Disinfectant validation
Posted: 3 December 2008 | Darren Wallis, Quality Assurance Microbiologist, GlaxoSmithKline | 1 comment
The design, validation and implementation of a documented and approved disinfectant programme must form a key part of any pharmaceutical production area qualification. There is significant regulatory interest in this area as it forms a fundamental part of any production facility maintenance schedule. European pharmaceutical companies are required to implement the necessary measures in order to comply with the requirements set out in EudraLex Volume 14 of the “Rules Governing Medicinal Products in the European Union”1. These guidelines are more commonly known as the EU Guide on GMP (EU-GMP). Pharmaceutical companies who supply to the United States are also required to comply with the GMP requirements of 21 CFR 211.56 “Sanitation” and 21 CFR 211.67 “Equipment cleaning and maintenance”.
The design, validation and implementation of a documented and approved disinfectant programme must form a key part of any pharmaceutical production area qualification. There is significant regulatory interest in this area as it forms a fundamental part of any production facility maintenance schedule. European pharmaceutical companies are required to implement the necessary measures in order to comply with the requirements set out in EudraLex Volume 14 of the “Rules Governing Medicinal Products in the European Union”1. These guidelines are more commonly known as the EU Guide on GMP (EU-GMP). Pharmaceutical companies who supply to the United States are also required to comply with the GMP requirements of 21 CFR 211.56 “Sanitation” and 21 CFR 211.67 “Equipment cleaning and maintenance”.
The design, validation and implementation of a documented and approved disinfectant programme must form a key part of any pharmaceutical production area qualification. There is significant regulatory interest in this area as it forms a fundamental part of any production facility maintenance schedule. European pharmaceutical companies are required to implement the necessary measures in order to comply with the requirements set out in EudraLex Volume 14 of the “Rules Governing Medicinal Products in the European Union”1. These guidelines are more commonly known as the EU Guide on GMP (EU-GMP). Pharmaceutical companies who supply to the United States are also required to comply with the GMP requirements of 21 CFR 211.56 “Sanitation” and 21 CFR 211.67 “Equipment cleaning and maintenance”.
This article will discuss the key industry standards and guidelines and highlight their significance within the pharmaceutical industry. The tasks that should be considered in order to validate your disinfectant products and cleaning programme will be outlined.
Definitions and terminology for antimicrobial products
Within the European Union (EU) and also the United States (US), there are a number of definitions and terms that are used to describe public health antimicrobial products that are used on inanimate objects and surfaces.
In the US, they are collectively known by the Environmental Protection Agency (EPA) as Antimicrobial Pesticide Products2.
In general, they are all substances or mixtures (blends) of substances that are used to destroy or suppress the growth of certain types of microorganism.
The following definitions are used in both the EU and the US:
Disinfectant
A chemical (or physical) agent that is used on hard inanimate surfaces such as walls, floors and other surfaces to destroy or inactivate bacteria and fungi but not necessarily their spores. They are often referred to as low or medium level disinfectants, depending on their level of activity.
Sporicides
Often referred to as high level disinfectants, they are used to destroy all forms of microbial life including viruses, fungi, bacteria and also low levels of their spores. A high level disinfectant can only be classed as a sterilant if it is capable of destroying all microorganisms present including high levels of spores.
Registration of antimicrobial products
In Europe, the registration of disinfectants is regulated by the 98/8/EC Directive known as the Biocidal Products Directive (BPD)3. The legislation which came into force in September 2000 outlined a plan lasting up to ten years that was designed to harmonise the manufacture and supply of biocidal products within the European Union.
The principle aim is to ensure that all biocidal products marketed within the European Union are safe, effective and non-hazardous to the environment. Within the scheme, biocidal products are divided into four main groups4. Disinfectants will fall into Main Group 1 “Disinfectants and General Biocidal Products”.
The product dossiers for the active ingredients that were notified under the scheme are now under evaluation. Each product that is authorised for use within the EU will be added to the approved list known as “Annex 1”5. Ultimately, only those actives contained in Annex 1 will be approved for use in individual product formulations which will be assessed in the next phase of the scheme.
Some active ingredient manufacturers opted not to include their products in the BPD scheme because the cost of the registration process was deemed to be too high for the potential financial gains. As a result of this, some disinfectant formulators were then forced to remove products containing these actives from the market or to reformulate the products. Products containing actives that were not deemed to be safe or were damaging to environment also had to be removed.
Manufacturers of chemical substances who produce or import more than one tonne per year of the chemical may also be required to register the chemical under the EC1907/2006 Regulation6. This new European Community law is known as REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) and came into force on 1 June 2007. It was designed to improve the protection of health and also the impact on the environment of chemical substances. The scheme is currently only in the early registration phase and it will therefore be some time before the benefits are fully realised.
In the US, the manufacture and sale of disinfectants (known as antimicrobial pesticide products) is regulated by the EPA under the statutory authority of the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA) amended in 19967.
In order to market a disinfectant in the US, the active ingredient must be registered with the US EPA under Title 7 of the FIFRA and 40 CFR Parts 152 and 1568. Disinfectants that are used on medical devices are regulated by the US Food and Drug Administration (FDA) under 21 CFR 880.6890, general purpose disinfectants and 21 CFR 880.6885, liquid chemical sterilants/ high-level disinfectants.
The test requirements for the product will be determined by the types of micro-organisms that it will be required to destroy9. The manufacturer must submit efficacy test data to support the label claim for the activity of the product.
Companies wanting to market their products within the EU and the US are required to submit dossiers to support their products under both the BPD and EPA schemes.

Efficacy testing of disinfectants
Although we often use the terms disinfectant efficacy testing and disinfectant validation in the same context, it is very important to make a distinction between these two terms.
Disinfectant efficacy testing is concerned with demonstrating that a product possesses antimicrobial activity under defined laboratory test conditions. It is the process that is used to compare the antimicrobial activity of a product against other products or known standards.
Disinfectant validation should be viewed as a form of process validation and is a much more in depth and extended process. It is often site or facility specific. In summary:
“Disinfectant validation is the documented verification and implementation of procedures that have been shown to consistently control the range and levels of micro-organisms that may be encountered on the surfaces in a facility”.
We will discuss the steps that should be considered during a disinfectant validation programme in more detail later. We will first consider the efficacy tests that must be performed by the suppliers of the disinfectants to gain approval for their products.
Under both the BPD and EPA schemes, manufacturers of disinfectants are required to submit efficacy data to support the antimicrobial claims for their product.
Within the EU, there have been a number of test methods published over the years by individual competent bodies. The French AFNOR method and German DGHM method are just a couple of examples.
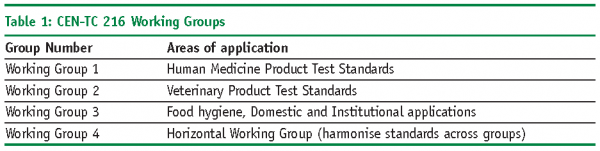
The European Committee for Standardisation (CEN-Comité Europeén de Normalisation) set up a group known as Technical Committee (TC) 216 in 198910. The aim of this group was to harmonise the approach used for the efficacy testing of disinfectants. There were four working groups (Table 1) set up to focus on different areas and applications.
The test procedures published by Working Group 3 (WG 3) are applicable to the pharmaceutical industry and have now been adopted by the British Standards Institution11 and published as BS EN standards. They consist of a phased test programme of suspension and surface tests using a range of standard test micro-organisms, they provide a useful framework of methods that can be used to verify the efficacy of the disinfectants under specific conditions.
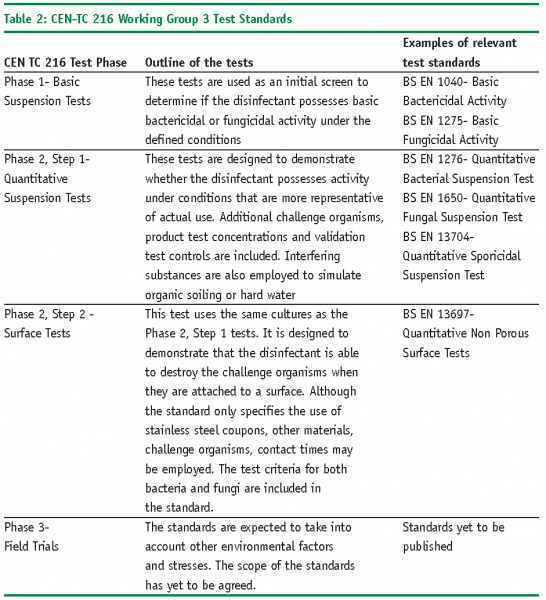

The following standards (Table 2) are examples of the tests used within the EU to verify the effectiveness of the disinfectants under the specified conditions.
In the US, it is stipulated by the EPA under subdivision G of the Pesticide Assessment Guidelines that effectiveness testing must be performed using methods that are accepted by the Association of Official Analytical Chemists (AOAC)12.
A limited efficacy claim for a “Germicide” or “Disinfectant” may be obtained by satisfying the criteria of the AOAC Use-Dilution Method (955.14, 955.15 and 964.02) (water soluble powders or liquid products) or the AOAC Germicidal Spray Product Test (961.02) (spray products). To obtain a claim for effectiveness against Gram-negative bacteria, this must be performed against Salmonella choleraesuis. To obtain a claim for activity against Gram-positive bacteria, this must be performed against Staphylococcus aureus.
A general purpose or broad spectrum efficacy claim would be required if the disinfectant must exhibit activity against a range of both Gram positive and Gram negative bacteria. This label claim may be obtained by satisfying the criteria of the AOAC Use-Dilution Method or the AOAC Germicidal Spray Product Test against both Salmonella choleraesuis and Staphylococcus aureus.
A hospital or medical disinfectant claim may be obtained by satisfying the criteria of the AOAC Use-Dilution Method or the AOAC Germicidal Spray Product Test against Salmonella choleraesuis, Staphylococcus aureus and also Pseudomonas aeruginosa.
There are individual AOAC tests for fungicidal activity (955.17), tuberculocidal activity (965.12) and sporicidal activity (966.04). The specific criteria in each test must be satisfied in order to obtain the relevant label claim.
The efficacy of disinfectants can be affected by a number of factors including pH, temperature water hardness, organic soiling and dilution. Many of these variables are taken into account in the BS EN and AOAC standards and specific test conditions are stipulated.
Disinfectant validation
We have already discussed the tests that may be used to verify the efficacy of the disinfectants under specific conditions.
These tests are very important because they determine the limitations of the disinfectant. Most importantly they help to establish the nominal microbial kill times that will be required during routine use. Useful information will be available from the suppliers because they will have generated this data as part of their product registration and development process.
Although the purchasers of disinfectants are under no obligation to use the BS EN (CEN 216) or AOAC tests, they do however provide a framework that can be used to devise efficacy tests that are representative of the end users’ requirements.
Some companies fail to obtain satisfactory disinfectant data through lack of awareness of the test standards and how to interpret the often variable data. Contract testing companies who perform these tests on a regular basis will be much more aware of the key requirements of the tests and how to interpret the data. They will be able to advise companies on the tests that would be required and more importantly, the tests or requirements that may not apply. Examples include the omission of the use of interfering substances or hard water if these conditions do not reflect the way the disinfectant would be used.
The United States Pharmacopoeia (USP) General Chapter 1072 “Disinfectants and Antiseptics”13, outlines the key tests that should be performed to verify the efficacy of the disinfectant against representative organism types and surfaces. It also highlights the physical and chemical factors that may influence the test results and challenge levels that should be employed.
The chapter stipulates that a 3 log reduction in the viable microbial count should be demonstrated for bacteria. It is worth noting that a 3 log reduction may have a very different meaning depending on the starting point. A 3 log reduction from 107 cells to 104 viable cells would constitute a relatively large reduction in the microbial cell count. However a 3 log reduction from 104 cells to 101 cells would constitute a much smaller reduction in the cell count. An analysis of the cell count reduction can therefore be very important.
Efficacy testing will be one of the key steps in the disinfectant validation process. However, you must also demonstrate that the procedures that are used to apply the disinfectants are able to routinely control the potential range of pathogens in the facility.
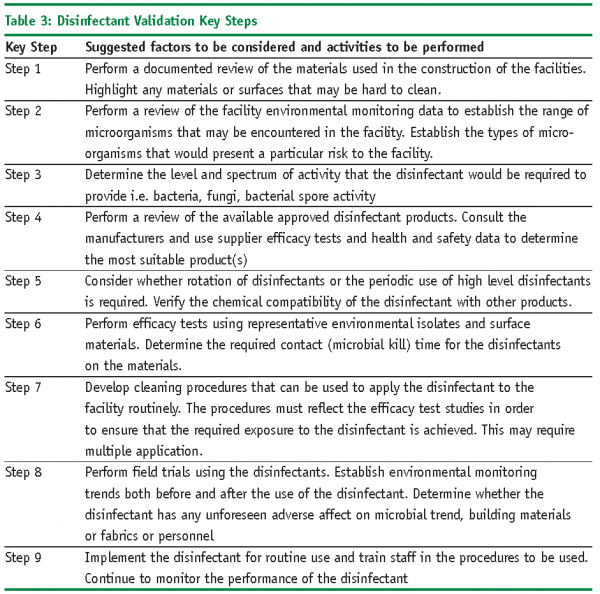

Table 3 provides an overview of the key steps that should be considered during a disinfectant validation programme:
The above list is not exhaustive. This is the type of process that should be followed to ensure that disinfectants are validated and implemented in a controlled and documented manner. There has been very little formal pharmacopoeial or industry guidance over the years regarding disinfectant process validation and how it should be conducted.
The regulatory agencies themselves have also been reluctant to enforce specific guidelines onto the industry due to the wide array of practices that are employed. However, numerous citations have been issued to companies for failing to provide adequate documented evidence or procedures to support their disinfectant programme.
Like other forms of process validation, disinfectant validation data must also be reviewed periodically to determine whether the original work is still representative of the current process. Factors that may drive revalidation include regulatory requests, changes in the microbial trend data or changes to the eqmaterials used in the manufacture of the facility if they were deemed to be significant. It would be at the discretion of the company to decide whether this was deemed appropriate or necessary.
Conclusions
The use of disinfectants will always be part of a pharmaceutical facility cleaning programme. Verifying that the routine disinfectant procedures are able to achieve control over the range of possible pathogens must always form a key part of the facility process qualification.
We have discussed the tasks that should be considered during a disinfectant validation programme. The responsibilities placed on the manufacturers to provide supporting data and the importance of ensuring that the overall validation reflects the way the products are used has also been highlighted. Validation does not have to be done in isolation and support and advice is widely available to ensure that it is performed to a satisfactory standard.
The risk associated with not performing these studies far outweighs the cost of performing them. Pharmaceutical facilities must be kept clean and under microbial control in order to protect the integrity of the products and ultimately the safety of the patients.
References
- European Commission-Enterprise and Industry-Pharmaceuticals (http://ec.europa.eu/enterprise/ pharmaceuticals/eudralex/vol4_en.htm)
- US Environmental Protection Agency, Antimicrobial Pesticide Products. (www.epa.gov/pesticides)
- European Chemicals Bureaux (ECB), (http://ecb.jrc.it/biocides/)
- European Commission (http://ec.europa.eu/environment/biocides/ scope.htm)
- European Commission (http://ec.europa.eu/environment/biocides/ basic.htm)
- European Commission (http://ec.europa.eu/environment/chemicals/reach/reach_intro.htm)
- US Environmental Protection Agency, Region 5 Information. (www.epa.gov/region5/defs/html/ fifra.htm)
- US Environmental Protection Agency, Pesticides: Regulating Pesticides (www.epa.gov/oppad001/regpolicy.htm)
- US Environmental Protection Agency, Pesticides: Science and Policy (www.epa.gov/oppad001/sciencepolicy).
- European Committee for Standardization (www.cen.eu/cenorm)
- British Standards Institution (BSI): Standards and Publications (www.bsi-global.com)
- Official Methods of Analysis, Association of Official Analytical Chemists (AOAC) (http://eoma.aoac.org)
- USP General Chapter <1072> “Disinfectants and Antiseptics”, Page3792-3795 (www.usp.org)






How to calculate log reduction