Advanced Aseptic Processing: RABS and Isolator Operations
Posted: 2 August 2008 | Eric A. Isberg, Product Manager, Bosch Packaging Technology | No comments yet
Advanced Aseptic Processing (AAP) is a term referenced in the recently published ISPE RABS definition1 to cover the spectrum of Restricted Access Barrier Systems (RABS) and isolator systems. In general, AAP techniques are physical barrier methods of product protection and containment that are used during manufacturing operations to separate (primarily) operators from the process. These methods are most often used during open processes or other critical process steps to ensure the product is not exposed to viable organisms and particulate contamination. While there are many methods to choose from, there is no argument that AAP techniques are widely used.
Advanced Aseptic Processing (AAP) is a term referenced in the recently published ISPE RABS definition1 to cover the spectrum of Restricted Access Barrier Systems (RABS) and isolator systems. In general, AAP techniques are physical barrier methods of product protection and containment that are used during manufacturing operations to separate (primarily) operators from the process. These methods are most often used during open processes or other critical process steps to ensure the product is not exposed to viable organisms and particulate contamination. While there are many methods to choose from, there is no argument that AAP techniques are widely used.
Advanced Aseptic Processing (AAP) is a term referenced in the recently published ISPE RABS definition1 to cover the spectrum of Restricted Access Barrier Systems (RABS) and isolator systems. In general, AAP techniques are physical barrier methods of product protection and containment that are used during manufacturing operations to separate (primarily) operators from the process. These methods are most often used during open processes or other critical process steps to ensure the product is not exposed to viable organisms and particulate contamination. While there are many methods to choose from, there is no argument that AAP techniques are widely used.
Complete and absolute ingress control is essential to AAP operation, and is essential to ensure an improvement over traditional open cleanroom processing. Operations within equipment like Laminar Flow Hoods (LFHs) or Biosafety Cabinets (BSCs) do not fit within the definition of AAP, as this equipment only provides partial separation (i.e. the hands and arms of the operators are not physically separated from the process when using this equipment). Curtained cleanroom areas are also not within this definition, as curtains provide little real barrier from ingress. Only complete, rigid wall enclosures fall within the scope of AAP.
The focus of this article is to compare the use of RABS and isolators for product final fill operations. It will give a general overview of both systems. It will then describe key mechanical and operational areas in which the two systems differ, while at the same time highlighting several key operational areas where they do not. Finally, it will propose that either system, if operated correctly according to approved procedures, will work successfully to ensure an improvement over traditional open cleanroom processing.
RABS and isolator overview
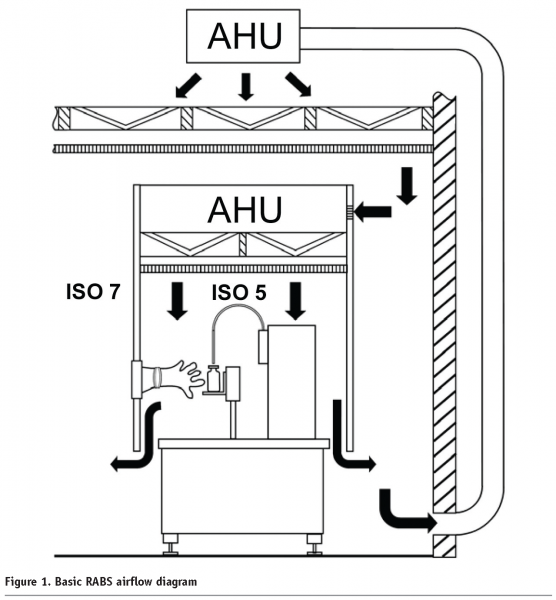
The ISPE RABS definition1 describes the common characteristics of a RABS system. The system has an ISO Class 5 environment2 with unidirectional airflow enclosed in a rigid wall enclosure with glove port access where necessary. The interior of the enclosure is manually sanitised with sterilised equipment and parts introduced using aseptic procedures which can include transfer systems. Because the system is open to the surrounding room, it is commonly located in an ISO Class 7 or better environment.2 All product or process contact parts within a RABS are sterilised or Steamed-In-Place (SIP) prior to use. Although doors can be opened, this happens rarely, after which appropriate line clearance and cleaning must occur per procedures.
Closed RABS, like isolators, fully enclose and seal the work area, and supply the interior with HEPA filtered air that is returned through sealed ductwork. The main difference between closed RABS and isolators is that closed RABS have no automated bio-decontamination cycle using H2O2 vapour or another sanitant. The interior of the closed RABS unit is bio-decontaminated manually using cleaning solutions. One purpose for closed RABS units is for highly potent compounds, where personnel protection is the purpose for product containment. In this case, they are designed as containment RABS, which require special leak tightness requirements, air filtration systems, and decontamination processes for safe operation.
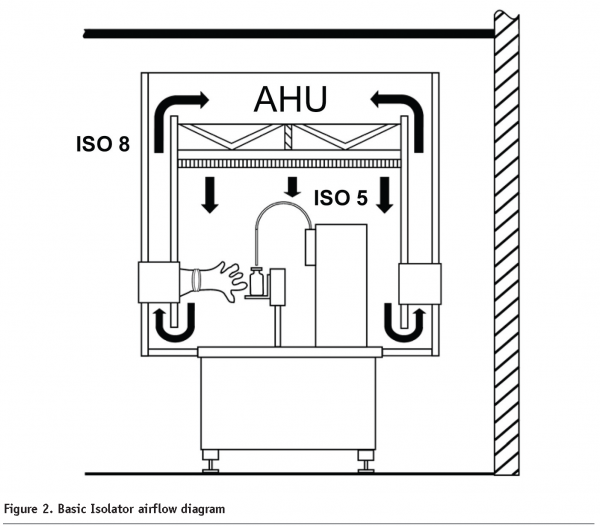
Isolators are fully enclosed and sealed units with HEPA filtered air supplied in a unidirectional manner to the ISO Class 5 interior2. Completely closed isolators may be supplied with either turbulent air or unidirectional air. Air is typically recirculated by returning it to the air handlers though sealed ductwork. The chamber is bio-decontaminated via an automated cycle using H2O2 or another sanitant. All access is through glove ports and sterile transfer systems. All items entering the system after bio-decontamination are pre-sterilised. Because they are sealed, isolators are commonly located in ISO Class 8 environments2.
Air Handling Differences
RABS systems operate in a similar fashion as LFHs in that they are fed clean air from fan units through HEPA filters and the air vents from the unit into the surrounding room (see Figure 1). The air is unidirectional via diffuser panels and multiple fan/HEPA filter locations. Therefore, the air handling requirements are relatively uncomplicated. Pressure balancing involving supply and return ductwork and return fans are not required. The exception to this is closed RABS, which can have a pressure differential to the outside room and therefore behave like an isolator in regard to basic air handling requirements.

Basic isolator air handling requirements are more complicated than RABS (see Figure 2). Air is re-circulated so return fans and ductwork is required. In order to maintain positive pressure, a large part of the air handling unit, including the return ductwork, must be leak tight. Leak testing of the system, which involves recording pressure decay over time, is required to prove the unit is sealed prior to sanitisation and operation. Ductwork that leads outside of the cleanroom, and outside of the building, is often required to safely vent H2O2 vapour after sanitisation where this chemical is used.

The bio-decontamination cycles for isolator units are mechanically complicated. For example, before injection of the H2O2 vapour (when used), the chamber and air handling ductwork must be conditioned. The purpose of the conditioning is to ensure a sufficient concentration of H2O2 vapour is injected into the system and that the H2O2 stays in vapour form during the cycle. Conditioning consists of heating the chamber and ductwork and lowering the humidity of the air in the system for low humidity bio-decontamination systems. High humidity bio-decontamination systems do not require humidity control during conditioning. After the bio-decontamination cycle is complete, out gassing of the H2O2 vapour is required to bring the concentration down to levels that are both safe for personnel, yet not high enough to affect the product being filled. Heating, Ventilation, and Air Conditioning (HVAC) systems are often required to perform these functions.
For smaller systems, the conditioning, bio-decontamination and aeration events can be performed using self-contained, external bio-decontamination systems. However, for larger isolator systems, multiple external bio-decontamination systems are often necessary, along with the HVAC system. These systems must be tied together to ‘act as one’ during bio-decontamination. One alternative is to have an integrated HVAC and bio-decontamination system. The advantage of this is that air handling, bio-decontamination, aeration and overall pressure balance can be controlled by the same unit.
Cleaning and bio-decontamination differences
The interior of isolators are bio-decontaminated using an automatic sequence which most often includes injection of H2O2 vapour as the sanitant. These cycles are very consistent and lead to a validatable bio-decontamination method. However, manual cleaning of the interior is still required on a regular basis. Direct contact cleaning is required to remove surface contaminants and to reduce the likelihood of biofilm formation. Also, manual cleaning is often required whenever the chamber is opened for parts changeover and other invasive events. Procedures should dictate when isolators are manually cleaned.
As described in the ISPE RABS definition1, the bio-decontamination of RABS units is not automatic. Manual spray and wipedown methods must be employed. The difficulty lies in performing consistent, repeatable and complete bio-decontamination using manual methods. Validation of the effectiveness of the cleaning and bio-decontamination solutions is an important step in justifying manual cleaning processes. In many cases, companies are trying to move away from manual cleaning and bio-decontamination because of consistency issues and the difficulty of validating manual methods. Alternatively, some companies offer periodic cleanroom bio-decontamination services using automated equipment that can be used for RABS systems.
Environmental monitoring
Environmental monitoring for both viable and non-viable particulates is key to determining classification levels in cleanroom space. Ongoing monitoring is required to show that the system maintains an ISO Class 5 environment over time2.
Isolator particulate and microbiological monitoring can only be achieved via built-in sampling ports or by transferring pre-sterilised sampling devices and sampling plates into the isolator in a sterile manner. Planning for environmental monitoring, including sampling methods, location of sampling devices and frequency of sampling must be a part of the isolator system design considerations.
Environmental monitoring via built-in sampling ports or by transferring pre-sterilised sampling devices and sampling plates can also be used for RABS. However, RABS units often have openings near floor level for air to flow out of the interior of the chamber. Therefore, there is the option of using portable sampling devices that have sampling probes that are inserted into these openings.
Personnel gowning
Operators must gown according to the classification of the area surrounding the AAP system. In the case of isolators, the gowning is often for an ISO 8 area.2 ISO 8 gowning is often comprised of items like a plant uniform or jumpsuit, lab coat, head cover and shoe covers. Single gloves are often used as a precaution during work using the isolator glove ports.
For RABS, the gowning must be for the ISO 7 or better environment2 where the equipment is located. This can include the addition of full, sterile one-piece suits, sterile face masks, sterile head and shoe covers, goggles and multiple layers of gloves. The personnel must still use glove ports when performing work within the RABS. Obviously, personnel comfort is a factor in RABS operation.
Glove replacement and testing
One area in which RABS and isolators do not differ is in the way that glove-port gloves and gauntlets are controlled. They must be sterilised prior to use, either by bio-decontamination or sterilisation processes. The gloves also require inspection prior to use, and periodic replacement is necessary to ensure effectiveness. A system of glove integrity testing and/or mechanical inspection may be required to provide evidence that leaks do not exist, as leaks can compromise the ISO Class 5 environments within the barrier system.
Built-in, automated glove testing systems are available with some RABS and isolator systems. These automated systems have the advantage of speed, as glove ports can be tested simultaneously and of accuracy compared to manual methods.
Procedural Requirements
A “paradigm shift” is required by an organisation to go from traditional open cleanroom processing to RABS and/or isolator use. All unit operations, including manufacturing, maintenance, quality, validation, and other functions, must prepare for this shift. Because processes will change and new processes will be required, Standard Operating Procedures (SOPs) must be created to govern them.
Adherence to new and revised SOPs is essential to ensure RABS and isolators are operated successfully. SOP requirements are more critical to RABS operation because there are more manual operations, such as the new bio-decontamination processes that will be required. However, if SOPs are written well and are followed by all personnel, either AAP method can be performed successfully.
Conclusions
Restricted Access Barrier Systems (RABS) and isolator systems are two methods of Advanced Aseptic Processing (AAP) that ensure an improved processing environment for cleanroom operations. Knowing some of the key mechanical and operational areas in which the two systems differ will increase awareness of the systems in the industry, and will help create a more detained definition of each.
These two AAP methods may, on first glance, appear to be very similar. Both methods provide ISO Class 5 cleanroom space and fully separate the operators from the process. However, of the two systems, only isolators are widely accepted within the industry for use in product fill operations. This is because RABS has been used in the past to label a wide variety of containment systems. Hopefully, the new ISPE RABS definition along with detailed AAP system comparisons like this one will help by increasing knowledge of how each system can be used successfully to ensure an improvement over traditional open cleanroom processing.
References
- Restricted Access Barrier Systems (RABS) for Aseptic Processing, ISPE Definition, 16 August 2005.
- International Standard ISO 14644-1:1999(E) Cleanrooms and Associated Environments – Part 1: Classification of air cleanliness
© ISPE 2007, Reprinted from with permission from Pharmaceutical Engineering, January/February 2007, Vol. 27 No. 1, www.ISPE.org