Wealth of worldwide evidence on Pradaxa® including first clinical data on long-term efficacy and safety of a novel oral anticoagulant to be presented
Posted: 31 October 2012 | | No comments yet
Results from RELY-ABLE®…


Widely anticipated results from RELY-ABLE®, the first long-term follow-up study of a novel oral anticoagulant treatment for stroke prevention in AF, will be among the numerous clinical reports on Pradaxa® (dabigatran etexilate) to be presented at the American Heart Association’s (AHA) Scientific Sessions in Los Angeles from 3-7 November.1 The wealth of evidence being presented for Pradaxa® at this year’s AHA sheds light on the clinical, long-term and real-world benefits of the treatment for patients at risk for thromboembolic events worldwide.
The RELY-ABLE® results presented are the first randomized follow-up data indicating how a novel oral anticoagulant is performing over more than four years of treatment duration.1 These important results will provide knowledge on the consistency of benefits offered by the treatment and support physicians in their search for optimal stroke protection. RELY-ABLE® is the long-term extension study of the RE-LY® trial, one of the largest phase III trials to investigate the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation.1-3
The RELY-ABLE® study conclusions will be discussed during an exclusive online data webcast available on demand for medical media from 9 a.m. CET on Thursday 8 November – www.newshome.com.
Additional studies on Pradaxa® being presented at the AHA Scientific Sessions
In addition to RELY-ABLE®, several reports including novel RE-LY® subanalyses, clinical real world experience and preclinical studies on Pradaxa® will be presented at the AHA Scientific Sessions. Of particular note will be results from real-world practice examining the safety and efficacy of Pradaxa® for the prevention of venous thromboembolism after total knee or hip replacement,4 as well as updates from the Boehringer Ingelheim Research & Development Programme regarding an antibody fragment antidote for rapid reversal of the anticoagulant effect of Pradaxa®.5
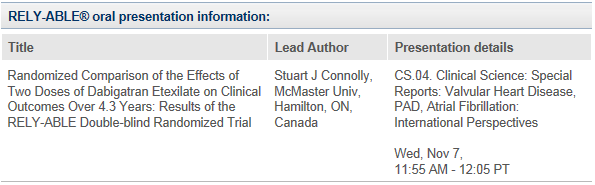

Further Pradaxa® abstracts being presented at AHA:*
ATRIAL FIBRILLATION
RE-LY® trial sub-analyses:
- Comparison of Dabigatran versus Warfarin in Diabetic Patients with Atrial Fibrillation: Results from the RE-LY Trial
Lead Author: H. Darius, Poster No. 15937, Sunday 4 November, 3:00 p.m. – 4:30 p.m. - Dabigatran Versus Warfarin in Very Elderly Patients with Atrial Fibrillation: Results from the RE-LY Trial
Lead Author: M. Coppens, Oral Presentation 15537, Monday 5 November, 9:30 a.m. – 9:45 a.m. - Balancing the Benefits and Risks of Two Doses of Dabigatran Compared with Warfarin in Atrial Fibrillation
Lead Author: J. Eikelboom, Presentation 14433, 11:30 a.m. – 11:45 a.m. - Importance of Persistent Elevation of Cardiac Biomarkers in Atrial Fibrillation – A RE-LY Substudy
Lead Author: Z. Hijazi, Oral Presentation 14808, Tuesday 6 November, 4:45 p.m. – 5:00 p.m.
Other key AF abstracts:
- Cluster Randomized Controlled Trial to Test The Effect of a Multifaceted Comprehensive Cardiovascular Care Intervention on Clinical Outcomes in Atrial Fibrillation Patients Receiving Dabigatran
Lead Author: R. Nieuwlaat, Oral Presentation 12618, Monday 5 November, 9:00 a.m. – 9:15 a.m. - Regional Variation in Ischemic Stroke Rates and Oral Anticoagulant Use Among the Non-Valvular Atrial Fibrillation Medicare Population
Lead Author: K. Fitch, Poster No. 12992, Tuesday 6 November, 9:30 a.m. – 11:00 a.m., presented by K. Siu - Association between Outpatient Visits Following Hospital Discharge and Readmissions among Medicare Beneficiaries with Atrial Fibrillation
Lead Author: M. Hubbard, Poster No. 11367, Tuesday 6 November, 9:30 a.m. – 11:00 a.m., presented by K. Siu - Incidence of Bleeding Events in Patients with Atrial Fibrillation
Lead Author: T. Murray-Thomas, Poster No. 14178, Tuesday 6 November, 3:00 p.m. – 4:30 p.m., presented by A. Clemens - Cost-Effectiveness of Dabigatran Etexilate versus Rivaroxaban for Stroke and Systemic Embolism Risk Reduction in Atrial Fibrillation: A US Third Party Payer Perspective
Lead Author: D. Walker, Poster No. 14964, Wednesday 7 November, 9:30 a.m. – 11:00 a.m.
Primary prevention of VENOUS THROMBOEMBOLISM in patients after total knee or hip replacement surgery:
- Safety and Efficacy of Once Daily 220 mg Dabigatran Etexilate in a Real-World Non-Interventional Study of More Than 5000 Patients After Total Knee or Hip Replacement
Lead Author: N. Rosencher, Poster No. 10001, 3:00 p.m. – 4:30 p.m.
PRE-CLINICAL STUDIES:
- The Influence on Plaque Formation and Endothelial Function in ApoE-Deficient Mice by Direct Thrombin Inhibition with Dabigatran
Lead Author: M.T. Kratz, Poster No. 14060, Sunday 4 November, 9:30 a.m. – 11:00 a.m. - Reversal of Anticoagulant Activity of Dabigatran and Dabigatran-induced Bleeding in Rats by a Specific Antidote (Antibody Fragment)
Lead Author: J. van Ryn, Oral Presentation 9928, Monday 5 November, 9:15 a.m. – 9:30 a.m. - Successful Reversal of Dabigatran-Induced Bleeding by 3-Factor Coagulation Concentrates in a Rat Tail Bleeding Model: Lack of Correlation with ex vivo Markers of Anticoagulation
Lead Author: J. van Ryn, Oral Presentation 11955, Monday 5 November, 11:30 a.m. – 11:45 a.m.
*Abstracts will be available on the AHA Scientific Sessions Website on Saturday, November 3, 2012
References
- Connolly S. J. et al. Randomized Comparison of the Effects of Two Doses of Dabigatran Etexilate on Clinical Outcomes Over 4.3 Years: Results of the RELY-ABLE Double-blind Randomized Trial. Presented on 7 November 2012 at the American Heart Association Scientific Sessions 2012.
- Connolly SJ, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139-51.
- Connolly SJ, et al. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363:1875-6.
- Rosencher N. et al. Safety and efficacy of once daily 220 mg dabigatran etexilate in a real-world noninterventional study of more than 5000 patients after total knee or hip replacement. Poster Presentation 10001. Presented on 5 November 2012 at the American Heart Association Scientific Sessions 2012.
- Van Ryn. J. et al. In vitro Chacterization, Pharmacokinetics and Reversal of supratherapeutic doses of dabigatran-induced bleeding in rats by a specific antibody fragment antidote to dabigatran. Oral Presentation 9928. Presented on 5 November 2012 at the American Heart Association Scientific Sessions 2012.
- Di Nisio M, et al. Direct thrombin inhibitors. N Engl J Med 2005; 353:1028-40.



