Roche’s obinutuzumab significantly reduced the risk of disease progression or death in people with one of the most common forms of blood cancer
Posted: 16 May 2013 | | No comments yet
First results from CLL11 announced…


Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced the first results from CLL11, a phase III study of the investigational medicine GA101 which is being conducted in collaboration with the German CLL Study Group (GCLLSG). The CLL11 study compared the combination of either GA101 or MabThera/Rituxan (rituximab) and chlorambucil, a standard chemotherapy, to chlorambucil alone in chronic lymphocytic leukemia (CLL). CLL is one of the most common forms of blood cancer and each year it causes approximately 75,000 deaths across the globe. The CLL11 study included elderly people with previously untreated CLL who were often not able to tolerate existing aggressive treatment options.
“People with CLL, particularly the elderly and those with additional medical problems, need new options,” said Sandra Horning, MD, Global Head, Clinical Development Hematology/Oncology. “As a former practicing hematologist, I believe GA101 has the potential to one day expand treatment options for people with CLL and we look forward to continuing to work with the FDA and health authorities around the world in an effort to bring GA101 to those in need.”
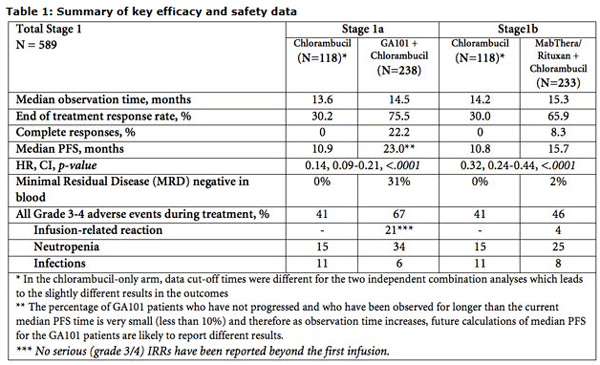
GA101 combined with chlorambucil demonstrated a significant 86% reduction in the risk of disease progression, relapse or death. Additionally, the length of time during which people lived without their disease worsening (median progression-free survival, PFS) was more than doubled (23 months compared to 10.9 months, HR=0.14, 95% CI 0.09-0.21, p <.0001) when compared to chlorambucil alone. The full data, including the comparison of MabThera/Rituxan plus chlorambucil with chlorambucil alone will be presented in an oral session at the 49th Annual Meeting of the American Society of Clinical Oncology (ASCO) in Chicago on Tuesday, June 4.
“Roche has played a significant role in revolutionizing the treatment of blood cancers. With GA101, our aim was to design a unique antibody that kills cancer cells directly and engages the patient’s own immune cells to help attack the cancerous cells,” said Pablo Umaña, Head of Roche Glycart AG.
GA101 is the first Type II anti-CD20 medicine that is glycoengineered, which means specific sugar molecules in GA101 were modified (using GlycoMAb technology) to change its interaction with the body’s immune cells with the goal of helping the immune system remove cancer cells from the body. In addition, as a type II anti-CD20 antibody, GA101 binds to CD20 with the aim of killing cancerous cells directly.
Based on the CLL11 data, marketing applications have been submitted to regulatory authorities including the European Medicines Association (EMA) and the U.S. Food and Drug Administration (FDA).
The FDA has granted GA101 ‘Breakthrough Therapy Designation’. This designation is designed to expedite the development and review of medicines intended to treat serious diseases and to help ensure patients have access to them through FDA approval as soon as possible.
About the CLL11 study
CLL11 is a phase III, multicenter, open-label, randomized three-arm study investigating the safety and efficacy profile of either GA101 added to chlorambucil or MabThera/Rituxan added to chlorambucil compared to chlorambucil alone in 781 previously untreated people with CLL and comorbidities (589 patients are included in this analysis and an additional 192 patients have been enrolled to enable the forthcoming direct comparison GA101 versus MabThera/Rituxan both in combination with chlorambucil). The study was conducted in collaboration with the German CLL Study Group (GCLLSG). The primary endpoint of the study was PFS with secondary endpoints including overall response rate (ORR), overall survival (OS), disease-free survival (DFS), minimal residual disease (MRD) and safety profile. Specifically, the CLL11 trial data to be presented during ASCO showed the following:
- The addition of GA101 to chlorambucil led to a statistically significant reduction in the risk of disease progression or death of 86 percent (HR=0.14; p-value=<0.0001).
- The median PFS improved by more than a year from 10.9 months for chlorambucil alone to 23 months for GA101 plus chlorambucil. (see ** in table 1 below)
- The addition of MabThera/Rituxan to chlorambucil significantly reduced the risk of the disease progression or death during study follow-up by 68 percent (HR=0.32; p-value=<0.0001).
- Median PFS was 10.8 months for chlorambucil compared to 15.7 months for MabThera/Rituxan plus chlorambucil.
- At this time, no formal comparison between the GA101 and MabThera/Rituxan arms can be made as the number of PFS events required for that formal analysis has not yet been reached.
- No new safety signals were detected for either GA101 or MabThera/Rituxan.The most common grade 3/4 adverse events (AEs) for GA101 were infusion-related reactions (IRRs) and low cell count of certain white blood cells (neutropenia). The incidence and severity of IRRs decreased dramatically after the first infusion and no serious IRRs have been reported beyond the first infusion. The most common adverse events are displayed in table 1 below.
- The most common AEs in the MabThera/Rituxan arm were infections and neutropenia and are displayed in table 1 below.

About GA101
GA101 is an investigational medicine that works with the body’s immune system and is designed to attack cells that have a certain marker on their surface. GA101 is currently being investigated in a large clinical program, including multiple head-to-head phase III studies versus MabThera/Rituxan in indolent non-Hodgkin lymphoma (NHL) and diffuse large B-cell lymphoma (DLBCL).
Roche Glycart AG is a wholly–owned, independent research unit, part of Roche Pharma Research and Early Development.
About Roche in hematology
For more than 20 years, Roche has been developing medicines that redefine treatment in hematology. Today, we’re investing more than ever in our effort to bring innovative treatment options to people with cancers of the blood.
In addition to GA101, Roche’s pipeline of potential hematology medicines includes two antibody-drug conjugates (anti-CD79b [RG7596] and anti-CD22 [RG7593]), a small molecule antagonist of MDM2 (RG7112) and in collaboration with AbbVie, a small molecule BCL-2 inhibitor (RG7601/GDC-0199).



