Essential techniques for development
Posted: 28 September 2006 | | No comments yet
Thermal analysis methods and coupled techniques are well established procedures in material science. Due to the different information delivered, thermal analysis methods are concurrent or complementary to other analytical techniques such as spectroscopy, chromatography, melting point determination, loss on drying, assay, for identification, purity and quantitation. They are basic methods in the field of polymer analysis and in physical and chemical characterisation of pure substances as well as for mixtures. They find best application for pre-formulation, processing and control of the drug product.
The introduction of sophisticated, automated, robust and sensitive instruments considerably increases the advantages of these methods. New horizons are open with the availability of combined techniques and microcalorimetry, an emerging technique now used routinely.
Since change of temperature and moisture occur by processing and storage, changes of the solid state may have a considerable effect on activity, toxicity and stability of compounds. Pharmaceutical industry is faced with the new challenges of quicker development and higher performance in terms of technology, reliability and up-scale in an international cGMP environment. Current requirements of the International Conference of Harmonisation for the characterisation and the quantitation of polymorphism in new entities1 re-enforce the position of thermal analysis and microcalorimetric techniques which can deliver the right information for the thermodynamic relationships between phases for the proper selection of salt and crystal form. The amorphous state is better understood and determinable. DSC purity analysis is a fast absolute orthogonal purity technique for organic compounds. Stability screening with microcalorimetry, phase diagrams for interaction with excipients enable the drug product to be designed.
What are the thermal analysis and calorimetric techniques?
When a material is heated or cooled, there is a change in its structure or composition. These transformations are connected with a heat exchange. The first method developed by Le Chatelier in 18872 was differential thermal analysis (DTA) where only the temperature induced in the sample was measured. Differential scanning calorimetry (DSC) which determines the heat flow into and out of the sample as well as the temperature of the thermal phenomenon during a controlled change of temperature is the basic thermal technique used for the industry. A typical DSC scan of an organic compound is exemplified in Figure 1.
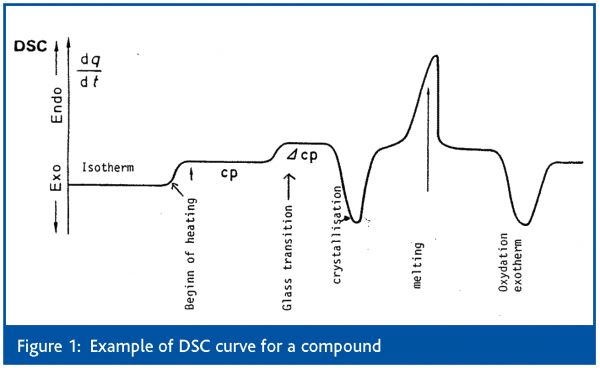

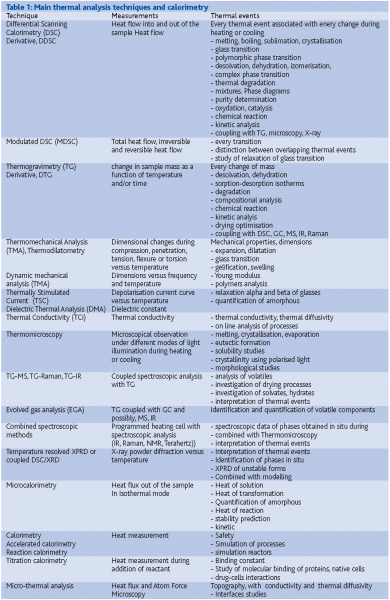
Thermal analysis techniques cover all techniques in which a physical property is monitored as a function of temperature or time while the sample is heated or cooled under controlled conditions. Table 1 summarises most methods covered including coupled and combined techniques.

Books or review articles dealing with the principle and instrumentation are given in References 3-6 and applications of thermal analysis methods for pharmaceuticals focused on industry are given in 7-20.
What are the areas of application?
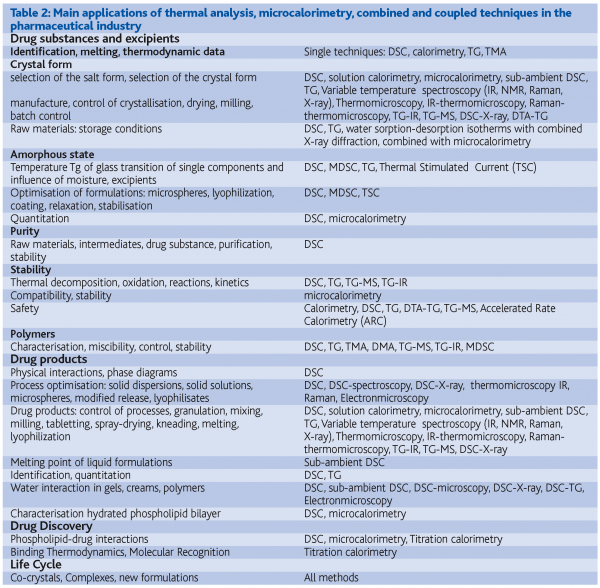
Table 2 summarises the main features of the methods and the huge areas of applications.

Thermal analysis and development
Manufacturing of pharmaceuticals generally implies two separated steps: manufacturing the active molecule (active pharmaceutical ingredient or drug substance) and manufacturing the formulation (dosage form or drug product) which is given to the patient. The formulation itself plays a decisive role for the action in the body, e.g. quick action, long action, site of action. According to the medical needs, the medicament can be applied orally as a tablet, a capsule, a syrup, a solution, or intramusculary or parenterally as injection, suppository, cream, gel, nasal spray or inhalation. Special delivery systems such as depot forms, minipumps and patches allow constant delivery for patients without the needs to be in hospitals. These formulations require auxiliary substances called excipients and adapted technologies for the manufacture of the drug product. For the patient it is mandatory that the product remains identical batch to batch and throughout its shelf-life in order to maintain the action at the desired time without unexpected side effects.

For mixtures of several components, the phase diagrams rules should be considered. If there is no interaction between two compounds in the solid state and miscibility of the liquid state, a eutectic behavior will be observed during heating of the mixture. This behaviour is the basis of the purity analysis by DSC and of the study of chiral purifications. If there is no interaction in the solid state and no miscibility in the liquid state, the DSC scan of the mixture of two compounds will be the addition of the two DSC scans. This is the basis of quantitation of components in the drug product. An interaction in the solid state may result from the formation of solid-solution with partial or total miscibility, from the formation of complex, or from chemical reaction. Pharmaceuticals may also be studied in aqueous media in order to follow, for example, protein denaturation, gel formation, liposome formation or in order to design the conditions of lyophilization.
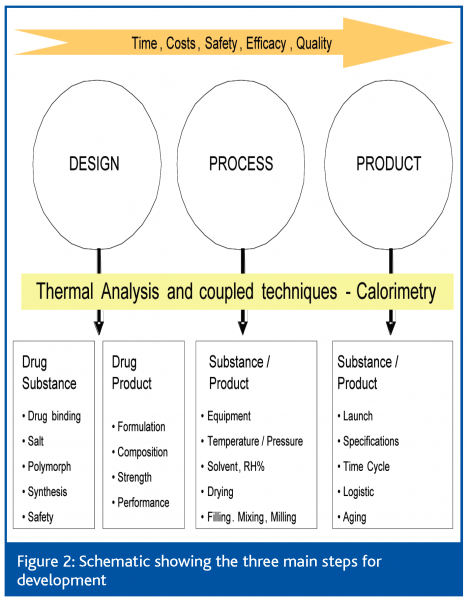
Figure 3 summarises the development steps- design, process and product- and the area of interest for the use of these techniques. The objectives are achieved by the proper choice of the salt form21, of the polymorphic form22-25, the control of the drug substance and of the excipients whatever their provenience as well as by the monitoring of the processing and the storage of the drug product.
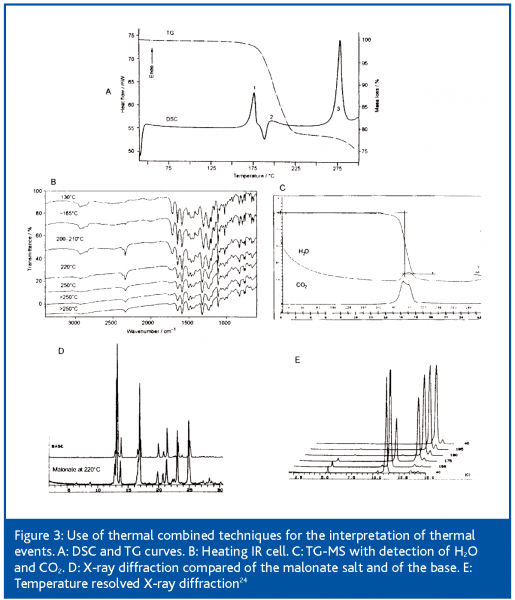

Figure 3 is an example of rational use of combined techniques for a proper and quick interpretation of thermal events. The DSC curve of a malonate salt would lead to the conclusion of a dual melting typical for polymorphism. After melting, malonic acid is decomposed and the drug substance recrystallises as base.
Thermodynamic and kinetic
The processing of the drug substances and drug products involve solvent(s), temperature and pressure changes as well as mechanical stress and different solid phases may coexist in the drug product. Organic substances show supersaturation behaviour and unstable solid phases which should not exist in defined temperature, pressure and humidity may behave like stable forms. These solid metastable phases obtained outside their domains of stability will convert to the thermodynamic stable forms at given temperatures, pressures and relative humidities in response to changes in environmental conditions, processing or over time. These conversions driven by thermodynamics are governed by kinetic and are influenced by impurities, particle size, crystal defects and presence of seeds.
The market withdrawal of ritonavir in 1998 – one year after its launch – was caused by its far too slow dissolution rate due to an insoluble stable polymorph not found during development. The current focus of research in the solid-state area is to understand the origin of polymorphism at the molecular level and to predict and prepare at the beginning of development the most stable form. The selection of a metastable form should result from targeted choice, not from the hazard. Thermal analysis techniques deliver basic thermodynamic rules for polymorphism22,23. The advantage of thermal and microcalorimetric techniques for their sensitivity allow the need to be significantly less than the 1 mg amount. For strategy of development and quantitation in drug substance and drug product, see references 23-25.
Combined thermal analysis techniques
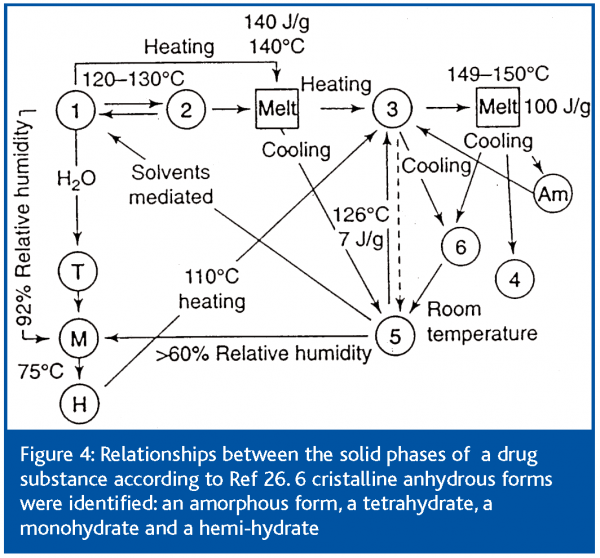
Figure 4 shows an example of polymorphism study for the relationships between all phases.

DSC Purity analysis
The method27 is especially attractive since it does not need a reference standard. The method delivers absolute purity or assay value. Results are obtained in less than 30 minutes.
DSC purity analysis allows the following:
- Optimisation of analytical stability indicative methods for drug substance
- Comparison of the quality of suppliers
- Optimisation of purification
- Comparison of methods for changes in synthesis
- Support results of routine batch analysis and stability studies
- Additional qualification of reference standards28
Study of the amorphous state
The use of amorphous forms is attractive, particularly for sparingly soluble compounds owing to the enhanced solubility and dissolution rate over the crystalline state, leading to increased bioavailability. Amorphous additives or excipients can give miscible phases with a new temperature of glass transition higher than the drug substance with the ability to stabilise it in the amorphous state. However, the amorphous state is thermodynamically unstable. The glass transition temperature, Tg, is lowered by water or other additives facilitating conversion to the rubbery state and hence facilitating crystallisation. Hancock and Zografi studied intensively the amorphous state of drug substances and used the relaxation energy at the glass transition as well as the dependency of the heating rate for the study of the ‘fragility’ of the amorphous state29. Stabilisation strategies have been researched30. More recently, Thermally Stimulated Depolarization Current (TSC) proved to be very sensitive for detection of the rigid fractions of the amorphous drugs and is used for the study of relaxation transitions alpha and beta31.
Microcalorimetry
Microcalorimetry is a growing technique32-38 complementary to DSC for the characterisation of pharmaceuticals. Larger sample volume and high sensitivity means that phenomena of very low energy (unmesurable by DSC) may be studied. The output of the instrument is measured by the rate of heat change (dq/dt) as a function of time with a high sensitivity better than 0.1 μW. Microcalorimetry can be applied to isolated systems in specific atmospheres; or for batch mode where reactants are mixed in the calorimeter. Solution calorimetry can be used in adiabatic or isoperibol modes in microcalorimeters at constant temperature. The method is used for polymorphic interpretation and for quantitation23. Microcalorimetry is useful for stability and compatibility predictions33-35.
The most useful application nowadays, thanks to high throughput microcalorimeters38, is the routine quantitation of amorphous content down to 0.1-0.3% when undesirable32-34,36-38,25.
References
- International Conference on Harmonization (ICH) (1999) Guideline Specification Q6A, Decision Tree: Investigating the need to set acceptance criteria for polymorphism in drug substances and drug products
- L. Le Chatelier, Compt. Rend., 104 , 1886. 1243.
- W.W.Wendlandt, Thermal Analysis, 3 rd Ed.. In Chemical Analysis ; Vol. 19., P. J.Elving, J. D.Winefordner, I. M Kolthoff, Eds ; John Wiley, New York, 1986.
- B. Wunderlich, Thermal Analysis, Academic Press, New York, 1990.
- P. J.Haines, Thermal Methods of Analysis, Principles, Applications and Problems, Blackie, Academic Professional, 1995.
- E.A. Turi, Thermal Characterization on Polymeric Materials ; 2nd Ed. ; Academic Press, New York, 1997.
- J.L. Ford and P. Timmins, Pharmaceutical Thermal Analysis Techniques and Applications, Ellis Horwood Books in Biological Sciences, Series in Pharmaceutical Technology, Rubinstein M.H., Ed., John Wiley&Sons, 1989.
- P.A. Barnes, Thermochim. Acta, 114, 1987, 1.
- D. Giron, J. Pharm. Biochem. Anal., 40,1986,755.
- D. Giron, Acta Pharm. Jugosl., 40,1990, 95.
- G. Bettinetti, Farmaco, 47 ,1992, 681.
- A.F. Barnes, M. J. Hardy and T.J. Lever, J. Therm. Anal., 40, 1993, 499.
- D. Giron, Thermal Analysis of Drugs and Drug Products, in Encyclopedia of Pharmaceutical Technology, 1995, vol. 15, p.1-79, Swarbrick, J. and Boylan, J.C., eds, Marcel Dekker.
- P.H. Willcoks, Thermochim. Acta, 256, 1995, 1.
- T. Wadsten, J. Therm. Anal., 47 ,1996, 525.
- D. Giron, C. Goldbronn, Use of DSC an TG for the identification and quantification of the dosage form, J. Therm. Anal. Calorim., 48,1997,473.
- D. Giron, J. Therm. Anal. Calorim., 56,1999,1285.
- D. Giron, Am. Pharm. Rev., 3, 2000, 3 and 3, 2000, 43.
- T. Ozawa, Thermochim. Acta, 355 ,,2000, 35.
- S.Z.D.Cheng, C. Y. Li, B.H. Calhoun, L. Zhu and W.W. Zhou, Thermochim. Acta, 2000, 355, 59.
- Giron, D.; Grant, D. J. W. in Handbook of Pharmaceutical Salts, ‘Properties, Selection and Use’,. P. H. Stahl,P.H; Wermuth, C.G. Eds, IUPAC, Wiley-Verlag Helvetica Chimica Acta, Zürich, 2002, Ch. 3 p.42-81
- Burger, A. and Ramberger, R., “On the polymorphism of pharmaceuticals and other molecular crystals. I. Theory of thermodynamic rules, Mikrochim. Acta [Wienn] , 1979, II, 259.
- Giron, D., Thermal Analysis and Calorimetric Methods in the Characterization of Polymorphs and Solvates, Thermochim. Acta, 1995, 248,1.
- Giron, D., J. Therm. Anal. Cal., 2001, 64, 37.
- Giron, D.; Garnier,S.; Mutz, M. J. Therm. Anal. Cal., 2004, 77, 709.
- D. Giron, M. Draghi, C.Goldbronn, S. Pfeffer and P. Piechon, J. Thermal Anal., 49 (1997) 913.
- D. Giron, C. Goldbronn, Place of DSC purity analysis in pharmaceutical development, J. Therm. Anal., 1995, 44, 217.
- D. Giron, C. Goldbronn, P. Piechon, Thermal Analysis methods for pharrmacopieal materials, J. Pharm. Biomed. Anal., 1989, 7, 1421.
- Hancock, B.C. and Zografi, G., (1997), “Characteristics and significance of the amorphous state in pharmaceutical systems”, J. Pharm. Sci, 1997, 86, 1-12 and Hancock, B.C; Shamblin, S.L. Thermochim. Acta, 2001, 380, 95-107
- Yu, L., (2001), “Amorphous pharmaceutical solids: preparation, characterization and stabilization”, Adv.. Drug Deliv. Rev., 2001, 48, 27-42.
- Correia, N.T.; Moura Ramos, J.J.; Descamps, M.; Collins, G., Pharm. Res., 2001, 18, 1767-1774
- M. Anberg, C. Nystrom and S. Carstensson, Int. J. Pharm., 81,1992,153.
- Buckton, P.Darcy, D. Greenleaf and P.Holbrook, Int. J.Pharm.,16, 1995,113.
- Gaisford, S., Buckton, G., Thermochim. Acta, 2001, 380, 185.
- Gaisford, S. Stability assessment of pharmaceuticals and biopharmaceuticals by isothermal microcalorimetry. Current Pharmaceutical Biotechnology, 2005, 6, 181-191.
- D. Giron, P.Remy, S. Thomas and E. Vilette, J. Thermal Anal., 48, 1997, 465.
- Garnier,S.; Giron,D.; Mutz, M., “Study of crystallization of drug substances under solvent vapour atmosphere by microcalorimetry”, PhandTA7, Innsbruck, september 2003
- Thermometric, Newletters and Application Notes. TAMIII, 48 channels. Sweden.




