DIFICLIR (fidaxomicin) significantly reduces recurrence and all-cause mortality when used first-line in all patients diagnosed with Clostridium difficile infection (CDI)
Posted: 21 May 2015 |
Data presented from the Clostridium difficile Infection (CDI) Service Evaluation study shows that the adoption pattern of treatment impacts CDI outcomes…

Data presented from the Clostridium difficile Infection (CDI) Service Evaluation study shows that the adoption pattern of treatment impacts CDI outcomes.

Compared to traditional broad-spectrum antibiotics, first-line use of fidaxomicin – a targeted treatment – in all CDI patients provides the best outcomes in terms of recurrence rate, all-cause mortality and cost effectiveness, compared to use in selected patients only. CDI is associated with high-mortality and cost burden, therefore reducing the incidence and recurrence of CDI is a priority for clinicians, payers and health authorities alike.
Presented at the 5th International Clostridium Difficile Symposium (ICDS), the CDI Service Evaluation Study is the first and only real-world multicentre study assessing the effectiveness of current CDI treatment in NHS Secondary Care Trusts in England.
“This study builds on the growing evidence that adopting fidaxomicin as first-line treatment for all patients with CDI, rather than reserving it for more severe cases, provides the best outcomes in terms of recurrence, all-cause mortality and cost effectiveness compared to older treatments – vancomycin and metronidazole,” commented Dr Simon Goldenberg, Consultant Microbiologist and Infection Control Doctor, Guy’s and St Thomas’ NHS Foundation Trust. “A previous study also showed that first-line use of fidaxomicin reduces environmental contamination compared to those treated with vancomycin or metronidazole, further demonstrating the role fidaxomicin may play in reducing the spread and incidence of CDI alongside stringent hospital hygiene protocols.”
Over 1,450 patients were included in the analysis conducted in seven UK hospitals that introduced fidaxomicin, a narrow-spectrum antibiotic for the treatment of CDI, between July 2012 and July 2013. Data collected from 177 patients treated first-line with fidaxomicin during the 12-month evaluation period were compared with those from a retrospective cohort treated with broad-spectrum antibiotics – vancomycin and metronidazole – during the previous 12-month period.
ESCMID identified recurrence as the next big challenge in the treatment of CDI
In the two centres (A and B) where fidaxomicin was adopted as a first-line treatment for all patients diagnosed with CDI, a significant reduction in 28-day all-cause mortality was observed, from 18.2% to 3.1% (P<0.001) and 17.3% to 6.3% (P<0.05) respectively. The real-world analysis also supports clinical trial data in highlighting dramatically reduced recurrence rates: from 12.1% and 23.5% with vancomycin and metronidazole, to 3.1% in both centres with first-line fidaxomicin. For every 50 patients treated, this would result in 5 and 10 recurrences avoided in the two centres respectively.
A separate study recently looked at the impact of CDI treatment on environmental contamination. The analyses showed those treated with fidaxomicin are more than 20% less likely to contaminate their environment with CDI (36.8%) compared to patients treated with metronidazole and/or vancomycin (57.6%). This significant decrease in environmental contamination may further contribute to a reduction in secondary cases of CDI.
“The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) identified recurrence as the next big challenge to be met in the treatment of CDI, since it occurs in up to 25% of patients treated with current broad-spectrum therapies,” commented Professor Mark Wilcox, Professor of Medical Microbiology, Leeds Teaching Hospitals & University of Leeds. “Fidaxomicin has limited activity against the ‘good bacteria’ in the gut and so can be considered to be a targeted treatment option. Preservation of the gut microflora likely contributes to the lower rates of recurrence seen after fidaxomicin treatment of CDI compared with those associated with broader-spectrum antibiotics like vancomycin.”
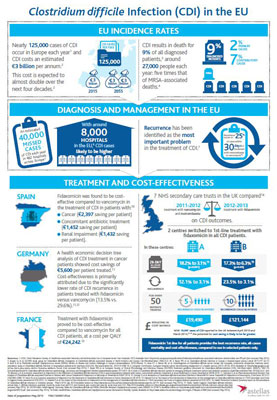
CDI recurrence is estimated to add an additional £20,249 on top of the amount spent to treat the initial infection
A CDI recurrence has been previously estimated to add an additional £20,249 on top of an estimated £13,146 spent to treat the initial infection due to prolonged hospital stay, ICU stay, high cost drugs and the surgery necessary to tackle it. An in-depth costing analysis at the two centres that adopted fidaxomicin as a first-line treatment revealed that in centre A the 5 recurrences that could be avoided for every 50 patients treated with the narrow-spectrum antibiotic would result in a cost saving of £19,490, and in centre B, for the 10 recurrences avoided, a cost saving of £121,144. With nearly 125,000 cases of CDI occurring in Europe each year, the potential cost saving for the treatment of this potentially fatal condition is likely to be far greater.
The cost-effectiveness of fidaxomicin has been reinforced in a recent study in France, with fidaxomicin proving to be both clinically and cost-effective compared to vancomycin. The main driver of cost-effectiveness was a significant reduction in the rate of recurrence, resulting in a reduced cost of hospitalisation. In the base case, fidaxomicin was cost-effective compared to vancomycin for all patients at a cost per QALY of €24,242. The cost per recurrence avoided was €1,877 and cost per faecal transplant avoided was €8,967.

CDI in the EU SOURCE: Astellas
Study author Dr David Jenkins said, “This study builds on the growing evidence that adopting fidaxomicin as first-line treatment for all patients with CDI, rather than reserving it for more severe cases, provides more optimal outcomes in terms of recurrence, all-cause mortality and cost effectiveness compared to older treatments – vancomycin and metronidazole.”
In Europe the incidence and severity of CDI is increasing, posing a major threat to healthcare systems and patients. Information suggests that CDI results in death for 9% (2% primary cause, 7% contributory) of all diagnosed patients. This suggests that CDI contributes to the death of around 27,000 people each year across Europe, around five times that of MRSA associated deaths.



