Polymorph screening in pharmaceutical development
Posted: 19 August 2010 |
The majority of active pharmaceutical ingredients (APIs) are produced by crystallisation and so the phenomenon of polymorphism, whereby an organic molecule can adopt more than one crystalline form (Figure 1), is of considerable importance when trying to achieve consistent product quality during the manufacture of pharmaceutical solids and solid dosage forms. Although morphology and particle size-distribution are important solid-state characteristics, the uncontrolled occurrence of multiple physical forms (polymorphs, solvates, salts, co-crystals or amorphous) of an API can have significant effects on the performance of the material during processing, manufacture, storage and administration. For example, the solubility difference between some polymorphs has been shown to be over four times that of the least soluble form1 and can vary by significantly more for amorphous forms2.
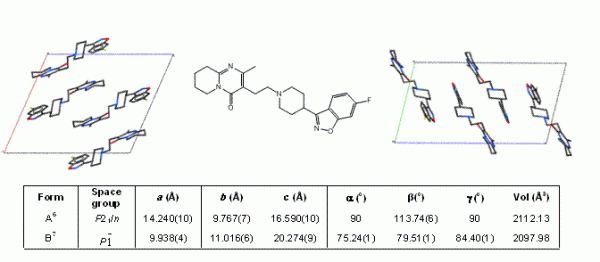

Figure 1 Packing diagrams showing the different molecular arrangements in polymorphs A (left) and B (right) of the antipsychotic drug risperidone (centre). Unit cell parameters for each polymorph are shown.
Such differences may have a potentially significant impact on the oral bioavailability of solid dosage forms or on the physical stability of liquid formulations or suspensions. For example, the unexpected appearance of a less soluble polymorph of the HIV-protease inhibitor ritonavir during production led to the product’s withdrawal from the market until a new formulation was developed (see reference 3 and references therein). Clearly, the potential clinical impact on patients of such events and the logistical, regulatory and financial consequences for manufacturers can be substantial and therefore justify the considerable investment in time and resources required to secure a robust understanding of the extent of physical form diversity for a new API and the factors that control it. The solid-state can also be an important aspect of API lifecycle management and intellectual property protection4,5. It is therefore necessary to obtain a comprehensive view of physical form diversity in a given API as quickly and efficiently as possible during the early drug development stages.
Crucially, predicting the occurrence and likely extent of polymorphism in a specific molecule, particularly with the conformational flexibility of a typical pharmaceutical molecule, is a significant challenge to theory. Whilst considerable advances have been made in the area of crystal structure prediction in recent years8,9, experimental polymorph screens are an essential component of pre-clinical drug development, informing the control of solidform throughout the subsequent scale-up, formulation, manufacture, storage and usage of pharmaceuticals10-12.
The interested reader is directed to a number of textbooks that give comprehensive coverage of the subject covering background, theory, experimental and analytical techniques and approaches for managing polymorphism in pharmaceuticals13-15. In this short article however, we shall highlight some of the experimental approaches that can be employed effectively to identify polymorphism in active pharmaceutical ingredients (APIs).
Crystallisation screening approaches
The fundamental objective in an experimental polymorph screen is to recrystallise the target API under as wide a range of conditions as can be achieved within the constraints of available compound, time and resources (Figure 2 )10. By selecting a sufficiently wide range of conditions under which nucleation and growth of API crystallites will occur, the aim is to sample a range of some, if not all, thermodynamic and kinetic solid products across the many individual crystallisations implemented. Solution crystallisation affords significant flexibility in terms of varying key parameters that influence nucleation16 and growth17 processes including solvent identity, super-saturation and agitation and is widely used in this context. It also has the advantage of being relatively straightforward to implement using automation and parallelisation, and a wide number of systems and approaches for parallel solution crystallisation have been described in the literature11,12,18-24. Whilst these systems vary in the extent of automation across all steps of the solution preparation → crystallisation → sample retrieval → analysis cycle, and in the scale of individual crystallisations carried out (e.g. from 96-well plates to ~5mL) and overall size of the screen (e.g. from a few 10s to 1000s of crystallisations), the basic process being performed is largely the same (Figure 3).
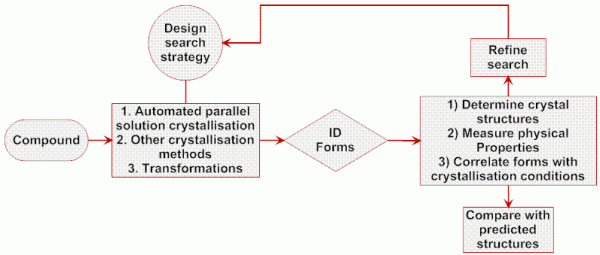

Figure 2 Schematic overview of an experimental screening strategy for polymorph discovery and control (see www.cposs.org.uk)


Figure 3 Schematic illustration of key stages and process variables included in an experimental polymorph screen using solution recrystallisation
In the early stages of development, only a few hundred milligrams of compound may be available for solid form screening and so small scale, high-throughput approaches using multi-well plates can prove to be very useful. For example, a high-throughput crystallisation search on an experimental angiotensin II antagonist revealed evidence for 18 crystalline forms, twice as many as were obtained using a traditional manual crystallisation approach19.
Typically, solid is dispensed in the form of a concentrated solution into individual wells and the solvent evaporated to leave the solid API ready for recrystallisation. Individual solvents or solvent mixtures can then be dispensed according to a predefined protocol from a library selected to cover a diverse range of physicochemical properties. Chemoinformatics, drawing on a range of multivariate statistical methods, can be used to quantify the diversity within the screening library and cluster solvents according to the extent of similarity and dissimilarity, thereby enabling the rational selection of solvents for use in the screen21,25-27. Once solvent is dispensed, plates can be warmed and agitated to aid dissolution before being subjected to a controlled cooling or evaporation cycle to achieve supersaturation and facilitate crystallisation. Optical inspection of samples between steps can identify wells where dissolution has not occurred prior to the crystallisation step. Once crystallisation has occurred, plates are passed for analysis in order to identify the solid form produced under each set of conditions tested. Detailed inspection of results can also be used to identify the experimental factors controlling polymorphic outcome from solution crystallisation28 and also to assess the completeness of the experimental screen29.
Where possible, multiple approaches to physical form discovery should be used to maximise the number of forms encountered during the search. Variations and alternatives to solution crystallisation that may be included as part of the comprehensive screening approach include (though are not limited to): contact line crystallisation30, crystallisation in constrained environments such as nanometer-scale pores in glass beads31, on self-assembled monolayers32 and in glass capillaries33, mechanically induced changes34, slurries24, heteroseeding35 and templating36, growth from the vapour phase via reverse sublimation37, polymer heteronuclei38 and microarrays39, crystallisation under high pressure40, in situ thermal transformations41, and recrystallisation from amorphous solids42.
Analysis and characterisation of solid forms
Whatever crystallisation method is used to generate solid samples of the API, once the formation of recrystallised solid is confirmed, the next step is to identify the form(s) produced. The primary analysis technique(s) used should ideally allow each sample to be identified unambiguously as comprising, for example, known or novel polymorphs, or a pure phase or a mixture of solid forms. A range of suitable analytical methods are listed in Table 1. Whilst many analytical techniques can be used very effectively to distinguish between different crystalline or amorphous forms of an API, crystallisation using high-throughput methods frequently results in very small amounts of recrystallised material deposited non-uniformly around the base or walls of the vessel. Locating the position of solid within each well introduces further challenges for the analysis of such samples. However, this can be achieved by analysis of multiple points across the well and/or using optical systems for location of material. When working at larger sample volumes (e.g. millilitre scale), sufficient sample may be produced to allow sample retrieval from the primary vessel by filtration prior to analysis. The speed of analysis is particularly important where metastable polymorphs or solvates have been formed that may transform relatively quickly to other forms that are produced in the search.
Table 1. Summary of analytical techniques for physical and structural analysis of polymorphs.
| Method | Comments | |
X-ray Diffraction | Single crystal |
|
| XRPD |
| |
Spectroscopy | Mid FT-IR |
|
| Raman |
| |
| Solid-state-NMR |
| |
Thermal analysis | DSC (differential scanning calorimetry) |
|
| TGA (thermal gravimetric analysis) |
| |
| Melting point |
| |
| Hot-stage microscopy |
| |
Other | Microscopy |
|
| DVS (dynamic vapour sorption) |
| |
| Solubility / Dissolution |
| |
| SEM (scanning electron microscopy) |
|
Raman spectroscopy43,44 (Figure 4) and X-ray powder diffraction (XRPD) utilising 2D area detectors12 (GADDS) are widely used, often in combination, to minimise the chance of missing or incorrectly identifying hits. Both techniques require no sample preparation, are able to collect data from very small individual crystallites and enable individual data sets to be collected very quickly (circa one minute). Where large numbers of samples are generated in the screen, multivariate data analysis tools can be essential for the reliable identification of all novel forms produced from the individual data sets collected45. Where larger sample amounts are available (e.g. 10 milligrams), other instrument configurations can be used to excellent effect46.
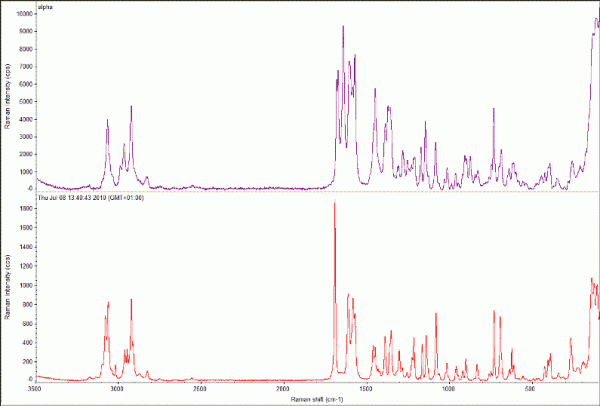

Figure 4 Raman spectra collected using a Thermo DXR Raman microscope from samples of the α47 (top; monoclinic, P21, a = 5.462 (2) Å; b = 25.310 (9) Å; c = 18.152 (7) Å, β = 94.38 (3)°) and γ48 (bottom;; triclinic, P⎯1, a = 9.236 (5) Å; b = 9.620 (5) Å; c = 10.887 (5) Å, α = 69.897 (5)o; β = 87.328 (5)o; γ = 69.501 (5)o) polymorphs of indomethacin. Data collected using 532 nm laser (10 mW) and 5 x 1s (α) and 10 x 5s exposures (γ). Insets, micrographs of each sample prior to data collection, images at 20 x magnification.
Once all forms produced in the screen have been identified, each one can be more fully characterised including determination of crystal structure and establishing thermodynamic relationships between forms and relative thermodynamic stabilities. Single-crystal diffraction is invaluable in the structural analysis of organic molecular crystals providing details on all of the atomic positions within the material including unit cell, space group, molecular conformation and intermolecular packing. These data provide a detailed description of each crystalline form. Single-crystal samples of at least 100 μm are typically required for structure determination using modern laboratory instruments; however, much smaller, weakly scattering crystals may also yield sufficiently good data to allow structure determination when data is collected using synchrotron sources49. Where suitable single crystal samples (or microcrystals) are not available for a specific API solid form, structure determination from powder data (SDPD) is a powerful alternative approach, requiring only a few milligrams of polycrystalline sample, good quality data (readily accessible using well calibrated laboratory instruments) and a knowledge of the chemical composition of the sample50. XRPD (Figure 5) is widely applied to the identification of polycrystalline powders produced during experimental crystallisation screening and in addition to potential application to SDPD studies, it can also provide information on whether the sample is crystalline or amorphous; whether it contains a single or mixture of crystalline phases; whether any known single-crystal structures are representative of the bulk material and, in combination with sample temperature control can allow direct study of structural transformations51. The application of synchrotron powder diffraction data to the structural characterisation of ‘X-ray amorphous’ samples using pair distribution function (PDF) analysis also shows considerable potential to further extend the role of powder diffraction in the development of pharmaceuticals52.
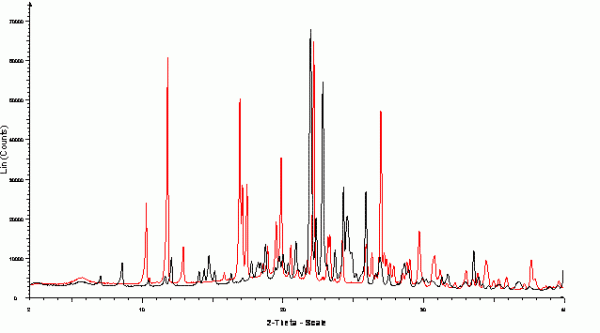

Figure 5 Capillary XRPD data collected from polycrystalline samples of α (black) and γ (red) polymorphs of indomethacin at 100K. Samples were lightly ground and filled into 1.0mm polyimide capillaries and were rotated during the data collection. Data collected in the range 2-40o 2θ on a Bruker-AXS D8 using capillary transmission geometry with primary monochromated CuKα1 (λ = 1.54056 Å) radiation and Lynxeye 1D position sensitive detector.
Summary
The general outline provided above illustrates the typical stages involved in the experimental assessment of polymorphism. Armed with this knowledge, subsequent stages are informed by a more complete understanding of the range of potential forms that may be encountered, their properties and the conditions under which they are formed and transform. The technologies that enable automated highthroughput polymorph screening to be carried out on small quantities of drugs have developed considerably in the last decade and the convergence of automation, experimental design, sensitive and rapid analytical techniques with sophisticated data analysis tools has led to a step-change in the rate at which physical form diversity for novel APIs is quantified and assessed. In addition, the process provides unambiguous fingerprint and structural data on forms, enabling better control over solid form as the product progresses through formulation, stability, scale-up and manufacturing stages.
Acknowledgements
Thanks to Rajni Miglani and Ryan Taylor for provision of Raman and capillary XRPD data.
References
- M. Pudipeddi and A. T. M. Serajuddin, Journal of Pharmaceutical Sciences, 2005, 94, 929-939.
- Bruno C. Hancock and Michael Parks, Pharmaceutical Research, 2000, 17, 397-404.
- S. L. Morissette, S. Soukasene, D. Levinson, M. J. Cima and O. Almarsson, Proceedings of the National Academy of Sciences of the United States of America, 2003, 100, 2180-2184.
- Joel Bernstein, in Polymorphism in the pharmaceutical industry, ed. Rolf Hilfiker, Wiley-VCH; [Chichester : John Wiley [distributor]], Weinheim, 2006, pp. 365-382.
- Rolf; Blatter Hilfiker, Fritz; von Raumer, Markus, in Polymorphism in the pharmaceutical industry, ed. Rolf Hilfiker, Wiley-VCH; [Chichester : John Wiley [distributor]], Weinheim, 2006, pp. 1-19.
- O. M. Peeters, N. M. Blaton and C. J. De Ranter, Acta Crystallographica Section C, 1993, 49, 1698-1700.
- Dan-Hua Wang, Ming-Hua Zhou and Xiu-Rong Hu, Acta Crystallographica Section E, 2006, 62, o3527-o3528.
- Sarah L. Price, Advanced Drug Delivery Reviews, 2004, 56, 301-319.
- S. L. Price, Accounts of Chemical Research, 2009, 42, 117-126.
- A. J. Florence, in Polymorphism in pharmaceutical solids, ed. H. G. Brittain, Informa Healthcare, New York ; London, 2009, pp. 139-184.
- R. Hilfiker, J. Berghausen, F. Blatter, A. Burkhard, S. M. De Paul, B. Freiermuth, A. Geoffroy, U. Hofmeier, C. Marcolli, B. Siebenhaar, M. Szelagiewicz, A. Vit and M. von Raumer, Journal of Thermal Analysis and Calorimetry, 2003, 73, 429-440.
- R. Storey, R. Docherty, P. D. Higginson, Dallman C., C. J. Gilmore, G. Barr and W. Dong, Crystallography Reviews, 2004, 37, 243-252.
- Joel Bernstein, Polymorphism in molecular crystals, Oxford University Press, New York; Oxford, 2002.
- H. G. Brittain, ed., Polymorphism in pharmaceutical solids, Informa Healthcare, New York, 2009. 15. Rolf Hilfiker, Polymorphism in the pharmaceutical industry, Wiley-VCH; [Chichester : John Wiley [distributor]], Weinheim, 2006.
- J. W. Mullin, in Crystallization, Butterworth- Heinemann, Oxford; Boston, 2001, pp. 181-215.
- J. W. Mullin, in Crystallization, Butterworth- Heinemann, Oxford; Boston, 2001, pp. 216-288.
- J. Aaltonen, M. Alleso, S. Mirza, V. Koradia, K. C. Gordon and J. Rantanen, European Journal of Pharmaceutics and Biopharmaceutics, 2009, 71, 23-37.
- O. Almarsson, M. B. Hickey, M. L. Peterson, S. L. Morissette, S. Soukasene, C. McNulty, M. Tawa, J. M. MacPhee and J. F. Remenar, Crystal Growth & Design, 2003, 3, 927-933.
- W. I. Cross, N. Blagden, R. J. Davey, R. G. Pritchard, M. A. Neumann, R. J. Roberts and R. C. Rowe, Crystal Growth & Design, 2003, 3, 151-158.
- M. Mirmehrabi and S. Rohani, Journal of Pharmaceutical Sciences, 2005, 94, 1560-1576.
- S. L. Morissette, O. Almarsson, M. L. Peterson, J. F. Remenar, M. J. Read, A. V. Lemmo, S. Ellis, M. J. Cima and C. R. Gardner, Advanced Drug Delivery Reviews, 2004, 56, 275-300.
- A. J. Florence, A. Johnston, P. Fernandes, N. Shankland and K. Shankland, Journal of Applied Crystallography, 2006, 39, 922-924.
- A. J. Alvarez, A. Singh and A. S. Myerson, Crystal Growth & Design, 2009, 9, 4181-4188.
- A. J. Florence, A. Johnston, S. L. Price, H. Nowell, A. R. Kennedy and N. Shankland, Journal of Pharmaceutical Sciences, 2006, 95, 1918-1930.
- D. Xu and N. Redman-Furey, International Journal of Pharmaceutics, 2007, 339, 175-188.
- C. H. Gu, V. Young and D. J. W. Grant, Journal of Pharmaceutical Sciences, 2001, 90, 1878-1890.
- J. F. McCabe, Crystengcomm, 2010, 12, 1110-1119.
- A. Johnston, B. F. Johnston, A. R. Kennedy and A. J. Florence, Crystengcomm, 2008, 10, 23-25.
- J. S. Capes and R. E. Cameron, Crystal Growth & Design, 2007, 7, 108-112.
- J. M. Ha, B. D. Hamilton, M. A. Hillmyer and M. D. Ward, Crystal Growth & Design, 2009, 9, 4766-4777. 3
- I. S. Lee, A. Y. Lee and A. S. Myerson, Pharmaceutical Research, 2008, 25, 960-968.
- L. J. Chyall, J. M. Tower, D. A. Coates, T. L. Houston and S. L. Childs, Crystal Growth & Design, 2002, 2, 505-510.
- M. R. Chierotti, R. Gobetto, L. Pellegrino, L. Milone and P. Venturello, Crystal Growth & Design, 2008, 8, 1454-1457.
- D. Braga, F. Grepioni, L. Maini, M. Polito, K. Rubini, M. R. Chierotti and R. Gobetto, Chemistry-a European Journal, 2009, 15, 1508-1515.
- C. Capacci-Daniel, K. J. Gaskell and J. A. Swift, Crystal Growth & Design, 2010, 10, 952-962.
- J. B. Arlin, A. Johnston, G. J. Miller, A. R. Kennedy, S. L. Price and A. J. Florence, Crystengcomm, 2010, 12, 64-66.
- C. P. Price, A. L. Grzesiak and A. J. Matzger, Journal of the American Chemical Society, 2005, 127, 5512-5517.
- A. R. Liberski, G. J. Tizzard, J. J. Diaz-Mochon, M. B. Hursthouse, P. Milnes and M. Bradley, Journal of Combinatorial Chemistry, 2008, 10, 24-27.
- F. P. A. Fabbiani, D. R. Allan, W. I. F. David, A. J. Davidson, A. R. Lennie, S. Parsons, C. R. Pulham and J. E. Warren, Crystal Growth & Design, 2007, 7, 1115-1124.
- A. J. Florence, K. Shankland, T. Gelbrich, M. B. Hursthouse, N. Shankland, A. Johnston, P. Fernandes and C. K. Leech, Crystengcomm, 2008, 10, 26-28.
- V. Andronis and G. Zografi, J. Non-Cryst. Solids, 2000, 271, 236-248.
- W. Paul Findlay and David E. Bugay, Journal of Pharmaceutical and Biomedical Analysis, 1998, 16, 921-930.
- T. Kojima, S. Onoue, N. Murase, F. Katoh, T. Mano and Y. Matsuda, Pharmaceutical Research, 2006, 23, 806-812.
- G. Barr, W. Dong and C. J. Gilmore, Journal of Applied Crystallography, 2004, 37, 658-664.
- A. J. Florence, B. Baumgartner, C. Weston, N. Shankland, A. R. Kennedy, K. Shankland and W. I. F. David, Journal of Pharmaceutical Sciences, 2003, 92, 1930-1938.
- X. M. Chen, K. R. Morris, U. J. Griesser, S. R. Byrn and J. G. Stowell, Journal of the American Chemical Society, 2002, 124, 15012-15019.
- P. J. Cox and P. L. Manson, Acta Crystallographica Section E-Structure Reports Online, 2003, 59, O986-O988.
- W. Clegg and S. J. Teat, Acta Crystallographica Section C-Crystal Structure Communications, 2000, 56, 1343-1345.
- W. I. F. David, K. Shankland, L. B. McCusker and Ch. Baerlocher, eds., Structure determination from powder diffraction data, Oxford University Press, Oxford, 2002.
- A. J. Florence, in Modern pharmaceutics, Volume One: Basic Principles and Systems, eds. A. T. Florence and J. Siepmann, Informa Healthcare, New York ; London, 2009, pp. 253-310.
- S. J. L. Billinge, T. Dykhne, P. Juhas, E. Bozin, R. Taylor, A. J. Florence and K. Shankland, Crystengcomm, 12, 1366-1368.
About the Author
Alastair J. Florence (AJF) is Professor of Pharmaceutical Science at the Strathclyde Institute of Pharmacy and Biomedical Science (SIPBS) in the University of Strathclyde, Glasgow UK. He runs the Solid-State Research Group within SIPBS which specialises in investigations of pharmaceutical solids including solid-state polymorphism, ranging from crystallisation and crystallisation screening, to phase transformations, through to crystal structure determination from single-crystal and powder X-ray diffraction data.
Issue
Related topics
HTS (High Throughput Screening), Polymorphism Screening, Raman Spectroscopy






