FDA approves Lilly’s Taltz for plaque psoriasis
Posted: 23 March 2016 | | No comments yet
Taltz (ixekizumab) is intended for adult patients who are candidates for systemic therapy, phototherapy or a combination of both…
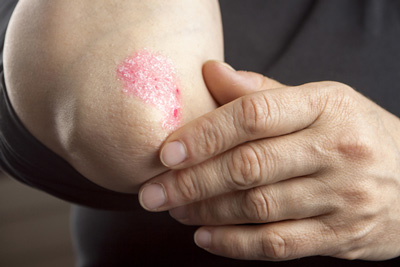

The US Food and Drug Administration (FDA) has approved Lilly’s Taltz (ixekizumab) for the treatment of moderate-to-severe plaque psoriasis in adult patients.


Taltz’s active ingredient is an antibody (ixekizumab) that binds to a protein (interleukin (IL)-17A) that causes inflammation. By binding to the protein, ixekizumab is able to inhibit the inflammatory response that plays a role in the development of plaque psoriasis.
Taltz is administered as an injection. It is intended for patients who are candidates for systemic therapy (treatment using substances that travel through the bloodstream, after being taken by mouth or injected), phototherapy (ultraviolet light treatment) or a combination of both.
Safety and efficacy of Taltz established in three trials
Taltz’s safety and efficacy were established in three randomised, placebo-controlled clinical trials with a total of 3,866 participants with plaque psoriasis who were candidates for systemic or phototherapy therapy. In all three studies, at 12 weeks, 87 to 90 percent of patients treated with Taltz saw a significant improvement of their psoriasis plaques (PASI 75). In addition, 81 to 83 percent of patients treated with Taltz achieved sPGA 0 or 1. The majority of patients treated with Taltz, 68 to 71 percent, achieved virtually clear skin (PASI 90) and 35 to 42 percent of patients saw complete resolution of their psoriasis plaques (PASI 100, sPGA 0). Among those patients treated with placebo, 7 percent or fewer achieved PASI 75, 7 percent or fewer achieved sPGA 0 or 1, 3 percent or fewer achieved PASI 90 and 1 percent or fewer achieved PASI 100 and sPGA 0.
Commenting on the approval, Alex Azar, president, Lilly USA, LLC, said: “Many people living with psoriasis are still looking for a treatment that will successfully manage the magnitude of this disease. With the approval of Taltz, we are proud to provide patients with a new treatment that may help patients experience virtually or completely clear skin.”
Julie Beitz, M.D., director of the Office of Drug Evaluation III in the FDA’s Centre for Drug Evaluation and Research, said: “Today’s approval provides patients suffering from plaque psoriasis with another important treatment option to help relieve the skin irritation and discomfort from the condition.”



