Development of stabilised vaccines with needle-free devices for targeted skin immunisation
Posted: 16 December 2010 | | No comments yet
Vaccination represents the primary public health measure to combat infectious diseases. However, limitations of cold-chain storage, vaccine wastage, hazardous sharps-waste and the requirements for trained personnel add significant and unsustainable financial and logistic costs to immunisation programmes. Developments of needle-free methods should aim to overcome these logistics issues from the very start of the vaccine production process.

Dermal vaccine administration using microneedle-based devices promises to be one such needle-free method that addresses all of these issues. Methods of stabilisation of vaccines onto or incorporated into microneedles should be developed to permit seamless transition and cost-effectiveness from vaccine bulk-up to final product. This review examines recent developments in microneedle technology and highlights the current challenges to translate this technology into practice.
Immunisation is the most successful strategy to combat infectious disease. However estimates of the cost of global immunisation programmes to 2015 demonstrate that up to 60 per cent of the total cost will be due to systems costs, predominantly cold chain, personnel and training1. Use of a simpler vaccine delivery device that eliminates sharps waste and reduces the requirements for training in correct vaccination technique as well as in appropriate waste handling would have a significant positive effect on the cost and success of immunisation. Another significant issue relating to vaccine deployment is the use of multi-dose vials. This can result in up to 50 per cent vaccine wastage; however the cubic volume of single dose vials (10 – 60 times greater than multi-dose vials) places a severe strain on cold-chain storage facilities1.
Vaccines are sensitive biological substances that can lose potency quickly if exposed to temperatures outside the recommended storage range. Unlike the majority of other pharmaceuticals, they consist of complex mixtures of carbohydrates, proteins, lipids and microorganisms (live or inactive) in addition to adjuvants, stabilisers and other preservatives. As part of the WHO’s Expanded Programme on Immunisation, major infrastructure, resources and human capital have been invested in monitoring and maintaining the temperatures to which vaccines are exposed. Some vaccines, such as the combination of diphtheria, tetanus and pertussis (DTP) and the Hepatitis B virus vaccine (HepB) are produced as liquid formulations and are resistant to elevated temperatures but can be quickly destroyed at freezing temperatures. This necessitates careful planning of where cold-sensitive vaccines are stored in comparison to vaccines that are more sensitive to increased temperatures.
In contrast, other injectable liquid vaccines that are not sufficiently stable for a shelf-life of two to three years in refrigeration are freeze-dried and shipped with a diluent vial for reconstitution. The dried state is preferable as removal of water is believed to retard degradative processes, but the drying process itself can induce significant changes in the vaccine components which contribute to instability and decreased vaccine yield. The requirement for reconstitution also increases the cost of packaging, requires extra syringes for reconstitution and increases the burden on the user to correctly store the different vials and as a consequence, it increases the rate of error of preparation.
Development of vaccine delivery strategies that overcome these obstacles is a key priority in global healthcare. Despite the drawbacks, stabilisation of vaccines into solid forms has the advantage of being adapted for novel routes or delivery devices such as inhalation or cutaneous delivery devices. A key challenge therefore is to develop processes that reduce or eliminate vaccine loss during its conversion to more stable forms that can be incorporated into needle-free devices that are easier to distribute and administer.
The skin as a target site for immunisation
The function of the skin, the largest organ in the body, is to protect against water loss and acts as the first line of defence against the entry of pathogens into the body. Mammalian skin can be subdivided into three layers; the stratum corneum (SC); in humans this is 10 – 20μm in depth, the viable epidermis (50 – 100μm in humans) and the dermis (one to three millimetres in humans)2 (Figure 1). The stratum corneum is composed of closely packed dead keratinocytes embedded in a highly organised extracellular lipid matrix composed of ceramides, cholesterol and fatty acids. This unusual matrix prevents the flux of hydrophilic toxins into the body. Delivery of material through this lipid bilayer system is highly anisotropic and size dependent, which has predominantly restricted transdermal drug delivery to lipophilic small molecules such as scopolamine, nitroglycerine or fentanyl. Recent drug delivery efforts have focused on enhancing transdermal delivery, either through chemical, electric or ultrasonic or iontophoretic means3,4.
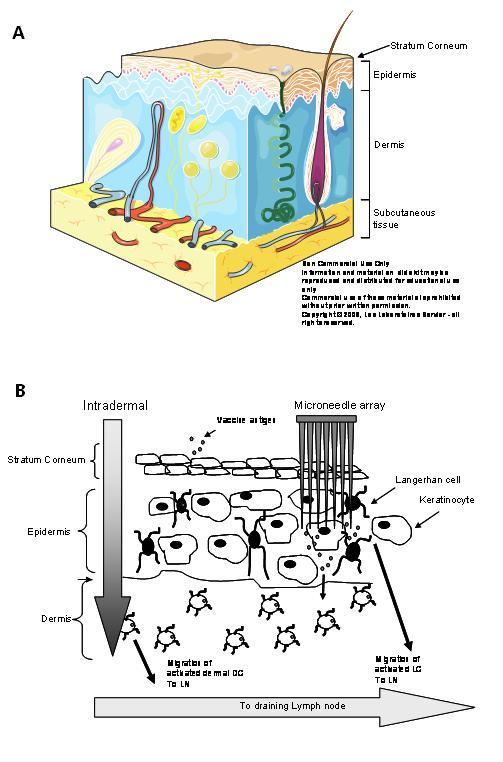

Figure 1 A – Anatomy of the skin; B – Vaccination through the skin requires breaching the stratum corneum and delivery either into the dermal space (intradermal) or into epidermal or dermal sites using microneedles
As part of its defence barrier function, the skin is an excellent site for immune targeting. A rich network of innate immune cells, such as Langerhans cells (LCs) and dermal dendritic cells (DC), reside in the underlying epidermis and dermis. The dramatic dose sparing effect that is evident with intradermal vaccination5 may be partly due to the efficient access of antigen by dermal immune cells, such as DC, in addition to the rapid drainage of dermally injected substances to the local lymph node6-8. Varying degrees of success with transcutaneous immunisation, using tape stripping, skin abrasion, targeting hair follicles and occlusive patches containing mucosal adjuvants have been achieved with several vaccines in the clinic9-12. Invasive particle based methods using gene guns have been developed to clinical trial using devices such as HeliosTM (BioRad), Accell® (Agracetus Inc.), PowderJect XR® (Novartis Vaccines). These function by delivering a powdered formulation into the LC-rich epidermis. Generally, DNA plasmid based vaccines coated on 1-2μm gold beads are delivered at high velocity, created by a burst of helium gas. Formulation of the vaccine with dense gold beads in conjunction with cumbersome, biolistic devices limits the cost-effectiveness and utility of these delivery systems to limited groups of trained healthcare professionals at this time13. Other invasive skin delivery technologies that are being developed to permeabilise the stratum corneum, such as iontophoresis, thermal microporation and photo – mechanisation require expensive or large equipment, preventing their use as mass vaccination devices or outside a research or specialised healthcare environment at this time13,14.
The perfect skin-based immunisation system would contain pre-packaged stabilised vaccine, preferably at lower effective antigen doses. Furthermore, its use would be pain-free and could be self-administered in an intuitive manner without the requirement for any supplementary equipment resulting in increased vaccine compliance. Such cost-effective, single-use devices would have a low cubic volume and the vaccine would be stable outside of cold chain conditions. This needle-free device would be paediatric friendly, capable of delivering routine immunisations as well as being quickly adapted for use with pandemic or bioterrorist vaccines. These attributes would have a significant impact on global health.
Microneedle vaccine delivery
Microneedle technologies are being investigated to satisfy all of these requirements. Microneedles are micron scale structures that are designed to pierce the stratum corneum and thereby permit drug and vaccine delivery to the epidermis and/or dermis. Due to their micron-sized height, they do not stimulate underlying nociceptors and have been shown to be painless in multiple human studies15,16. Many shapes and structures have been designed and fabricated from materials ranging from silicon to stainless steel to sugars and ranging in length from approximately 60 – 700μm that target either the epidermis or dermis, depending on increasing length. The majority of microneedles have been fabricated from silicon using techniques common to the semiconductor industry, such as deep reactive ion etching (DRIE) or wet-etching. The Tyndall National Institute optimised methods to fabricate wet-etched silicon microneedle arrays that have ultra-sharp tips that can penetrate skin at very low insertion forces (15mN per needle) (Figure 2). These Tyndall-fabricated micro needles can be safely and painlessly applied to human volunteers and can penetrate human skin, creating temporary micropores in the SC, with minimal pain and discomfort16,17.
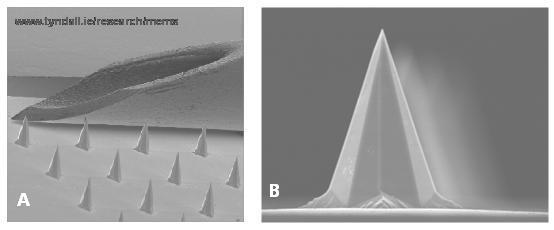

Figure 2 A – a scanning electron micrograph (SEM) that compares the bevel of a 26G needle to microneedles on a wet-etched Tyndall National Institute microneedle array device; B – SEM image of an individual microneedle
Four types of microneedle systems can be broadly categorised18; solid microneedles that pierce the skin to permit the drug or vaccine to diffuse past the SC, ‘coated microneedles’ where the drug or vaccine is coated in dry form onto the solid microneedle(s), ‘dissolvable microneedles’ where the microneedle structure itself is fabricated from biocompatible material that incorporates the vaccine and dissolves on contact with the warm, moisture-rich skin and finally hollow microneedles for injection. Of these categories, hollow microneedles have progressed the furthest to the clinic. Nanopass’s ‘MicronJet’ and Becton Dickinson’s Soluvia™ hollow needle intradermal delivery devices have been successfully tested or licensed for vaccine delivery, respectively. However neither of these devices is completely pain-free, cannot be self-administered and both of these products still rely on a liquid vaccine to be injected from a syringe through the microneedles, reducing the potential impact of these devices on overcoming immunisation logistics.
Formulation of vaccine onto or into microneedle arrays offers the most promising method of addressing key stability and administration issues. However, the micrometre lengths of microneedles and their close proximity to each other impose challenges on how to uniformly and efficiently coat these devices. This is largely due to the significant effects of surface tension, capillarity and viscous forces at these micron scales19. An important issue that has not been resolved is the orientation of the vaccine on the microneedle array. It has been postulated that vaccine coating of the tip of microneedles will blunt the sharp microneedle and result in decreased penetration of the skin. In contrast, coating the entire base of the array will likely waste material as vaccine on the base may not have access to the temporary pores in the skin created by the microneedle. We have observed that vaccine coated specifically around the base of microneedle shafts results in induction of immunity of equivalent magnitude to intradermal immunisation.
Many strategies of coating drugs or vaccines onto microneedles have been designed and published or patented. Current state-of-the-art in the coating of microneedles involves the use of specialised coating apparatus for dip coating20, rolling or brushing on the formulation21,22, or pattern coating using, for example, ink jet coating or microfluidics23 that require the use of wetting agents. The most common practice for coating microneedles is via dipping the microneedles into a drugcontaining reservoir that is covered to restrict the access of liquid only to the microneedle shaft. This and the roller-brushing method rely on varying the number of contacts (dips) between the microneedle and the reservoir or roller to control a dosage of biologically active compound to be coated on the microneedle. The idea of masking fluids was generated by 3M24,25; organic compounds that are more volatile than the coating fluid are first coated onto the microneedle arrays, this masks the base of the array from the coating formulation which is added on top, resulting in coated microneedle tips. While these methods may be highly suitable for drug loading, significant loss of vaccine stability has been demonstrated using these methods26.
Effective vaccine-loaded dissolving microneedles would be the most elegant solution to vaccine delivery problems. Due to the incorporation of the vaccine in the very device that penetrates and then dissolves in the skin, the issue of biohazardous waste is eliminated. Polymer microneedle fabrication has focused on dissolving biocompatible materials such as maltose, trehalose and other sugars, as well as poly-glycolic acid, polyvinylpyrrolidone (PVP), polyvinylalcohol (PVA) or carboxymethyl cellulose (CMC). They are generally fabricated by filling microneedle moulds using molten, vacuum or centrifugal casting techniques27-29. Key design characteristics that must be achieved when fabricating dissolvable microneedles include retention of vaccine antigenicity, structural strength so that the microneedle can penetrate the skin and resistance against humidity, which is often overcome by immediate hermetic packaging. Microneedle fabrication involving elevated temperatures such as molten processes to fabricate maltose microneedles is not a feasible vaccine-loaded microneedle process due to the thermolabile nature of most vaccines30. A recent elegant study29 fabricated dissolving polymer microneedles for flu vaccination by mixing the monomer and lyophilised vaccine in the microneedle mould and then initiating polymerisation. The inactivated flu vaccine retained its immunogenicity and efficacy against live virus challenge in BALB/c mice. Of interest, although these dissolving micro – needles were 650μm tall, they only penetrated to approximately 200μm and delivered the payload largely to the epidermis. This is likely due to the fast dissolving nature of the microneedle on contact with hydrated skin. It will be of interest to see if this in situ polymerisation technique will be applicable to a wide range of subunit and live vaccines that induce antibody and cell-mediated immunity.
It has been an exciting time for micro – needle-based vaccine delivery development, with the concept of dissolving vaccine delivery systems providing a solution to some of the barriers to immunisation campaigns becoming more of a reality. However, there are still many questions to answer before we achieve these goals. Vaccine-loaded microneedle fabrication methods should occur as early in the manufacturing process as possible. It should involve as few processing steps, such as freeze drying, as possible, thus minimising vaccine loss. Most current methods of fabricating coated and dissolving vaccine-loaded microneedles result in significant wastage of the active material. The re-use of excess material that has not coated or filled the microneedles29 needs to be addressed from a quality and regulatory viewpoint. Thus new methods of more precisely targeting precious cargo to its desired location on or in the microneedle are required. The choice of excipients used to formulate the vaccine onto microneedles will be crucial and may have to be made on a vaccine-by-vaccine basis. Formulations commonly used for microneedle coating that include surfactants and wetting agents decrease antigenicity very quickly26 and we have observed that such formulations can kill a range of live vaccines. If vaccine-loaded microneedles are to live up to current expectations, such changes in vaccine manufacturing should be made in the most cost-effective manner. We are addressing these issues by designing methods of vaccine-loaded microneedle fabrication, in vaccine-compatible formulations, that utilise commonly used scalable pharmaceutical methods and do not require investment in new untested instrumentation, equipment or methods.
Conclusions
Microneedles offer several advantages to deliver vaccine more safely and effectively and their successful development as needle-free, easy-to-administer, stable and cheap devices would address several logistic obstacles that are preventing immunisation programmes from reaching full potential. Identification of appropriate formulations for vaccine coating onto or incorporating into microneedle devices will be important to optimising the stability and potency of monovalent or multivalent vaccines in these devices. The complementary research efforts of engineers, pharmaceutical and immunological scientists, with appropriate discussion with relevant stakeholders, will be critical to successful development of this technology.
References
1. Wolfson, L. J., Gasse, F., Lee-Martin, S. P., Lydon, P., Magan, A., Tibouti, A., Johns, B., Hutubessy, R., Salama, P. & Okwo-Bele, J. M. (2008) Estimating the costs of achieving the WHO-UNICEF Global Immunization Vision and Strategy, 2006-2015. Bull World Health Organ 86, 27-39
2. Berman, B., Chen, V. L., France, D. S., Dotz, W. I. & Petroni, G. (1983) Anatomical mapping of epidermal Langerhans cell densities in adults. Br J Dermatol 109, 553-8
3. Cevc, G. & Vierl, U. (2010) Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J Control Release 141, 277-99
4. Prausnitz, M. R., Mitragotri, S. & Langer, R. (2004) Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 3, 115-24
5. Nicolas, J. F. & Guy, B. (2008) Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines 7, 1201-14
6. Harvey, A. J., Kaestner, S. A., Sutter, D. E., Harvey, N. G., Mikszta, J. A. & Pettis, R. J. (2010) Microneedle-Based Intradermal Delivery Enables Rapid Lymphatic Uptake and Distribution of Protein Drugs. Pharm Res
7. Gopee, N. V., Roberts, D. W., Webb, P., Cozart, C. R., Siitonen, P. H., Warbritton, A. R., Yu, W. W., Colvin, V. L., Walker, N. J. & Howard, P. C. (2007) Migration of intradermally injected quantum dots to sentinel organs in mice. Toxicol Sci 98, 249-57
8. Puri, N., Weyand, E. H., Abdel-Rahman, S. M. & Sinko, P. J. (2000) An investigation of the intradermal route as an effective means of immunization for microparticulate vaccine delivery systems. Vaccine 18, 2600-12
9. Vogt, A., Mahe, B., Costagliola, D., Bonduelle, O., Hadam, S., Schaefer, G., Schaefer, H., Katlama, C., Sterry, W., Autran, B., Blume-Peytavi, U. & Combadiere, B. (2008) Transcutaneous anti-influenza vaccination promotes both CD4 and CD8 T cell immune responses in humans. J Immunol 180, 1482-9
10. Glenn, G. M., Taylor, D. N., Li, X., Frankel, S., Montemarano, A. & Alving, C. R. (2000) Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat Med 6, 1403-6
11. Laurent, P. E., Bourhy, H., Fantino, M., Alchas, P. & Mikszta, J. A. (2010) Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine 28, 5850-6
12. Etchart, N., Hennino, A., Friede, M., Dahel, K., Dupouy, M., Goujon-Henry, C., Nicolas, J. F. & Kaiserlian, D. (2007) Safety and efficacy of transcutaneous vaccination using a patch with the live attenuated measles vaccine in humans. Vaccine 25, 6891-9
13. Chen, D., Maa, Y. F. & Haynes, J. R. (2002) Needle-free epidermal powder immunization. Expert Rev Vaccines 1, 265-76
14. Babiuk, S., Baca-Estrada, M., Babiuk, L. A., Ewen, C. & Foldvari, M. Cutaneous vaccination: the skin as an immunologically active tissue and the challenge of antigen delivery. J Control Release 66, 199-214 (2000)
15. Gill, H. S., Denson, D. D., Burris, B. A. & Prausnitz, M. R. (2008) Effect of microneedle design on pain in human volunteers. Clin J Pain 24, 585-94
16. Haq, M. I., Smith, E., John, D. N., Kalavala, M., Edwards, C., Anstey, A., Morrissey, A. & Birchall, J. C. (2008) Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices
17. Enfield, J., O’Connell, M. L., Lawlor, K., Jonathon, E., O’Mahony, C. & Leahy, M. (2010) In vivo dynamic characterization of microneedle skin penetration using optical coherence tomography (OCT). J. Biomedical Optics In press
18. Arora, A., Prausnitz, M. R. & Mitragotri, S. (2008) Micro-scale devices for transdermal drug delivery. Int J Pharm 364, 227-36
19. Gill, H. S. & Prausnitz, M. R. (2007) Coating formulations for microneedles. Pharm Res 24, 1369-80
20. Gill, H. S. & Prausnitz, M. R. (2007) Coated microneedles for transdermal delivery. J Control Release 117, 227-37
21. Trautman, J. C., Daddona, P. E. & Cormier, M. (2005) Apparatus and Method for Transdermal Delivery of Multiple Vaccines. WO2005/103303A2
22. Trautman, J. C., Wright, C. T. & Cormier, M. (2002) Method and Apparatus for Coating Skin Piercing Microprojections. WO2002/074173
23. Cormier, M., Young, W. A., Johnson, J. A., Daddona, P. E. & Armeri, M. (2009) Transdermal Drug Delivery Devices Having Coated Microprotrusions. US2009/0186147 24. Duan, D. C. & Johnson, P. R. (2007) Microneedle Arrays and Methods of Preparing Same. WO2007/059289A1 25. Wolter, J. T., Simons, J. K. & Johnson, P. R. (2007) Methods for Coating Microneedles. WO2007/061964 26. Kim, Y. C., Quan, F. S., Compans, R. W., Kang, S. M. & Prausnitz, M. R. (2009) Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release
27. Lee, J. W., Park, J. H. & Prausnitz, M. R. (2008) Dissolving microneedles for transdermal drug delivery. Biomaterials 29, 2113-2124
28. Park, J. H., Allen, M. G. & Prausnitz, M. R. (2005) Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. Journal of Controlled Release 104, 51-66
29. Sullivan, S. P., Koutsonanos, D. G., Del Pilar Martin, M., Lee, J. W., Zarnitsyn, V., Choi, S. O., Murthy, N., Compans, R. W., Skountzou, I. & Prausnitz, M. R. (2010) Dissolving polymer microneedle patches for influenza vaccination. Nat Med 16, 915-20
30. Donnelly, R. F., Morrow, D. I., Singh, T. R., Migalska, K., McCarron, P. A., O’Mahony, C. & Woolfson, A. D. (2009) Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev Ind Pharm 35, 1242-54
About the Authors
Dr. Abina Crean is a lecturer in pharmaceutics at the School of Pharmacy, University College Cork, Ireland. Her research interests focus on the design of drug delivery systems and in particular, the impact of processing on solid state form and biopharmaceutical stability.
Dr O’Mahony is a Staff Researcher at the Tyndall National Institute, Ireland, where he develops silicon micro – needle technology for a range of biomedical applications, including vaccine delivery, cancer treatment and ECG/EEG analysis. He has published over one hundred research papers and filed seven patent applications in the fields of microneedles and micromachining.
Dr. Anne Moore’s research focuses on developing and translating cost-effective vaccine technologies that induce cellular immunity against infection, particularly vaccines against viruses and intracellular organisms, such as malaria parasites and TB. Contact Anne at [email protected]