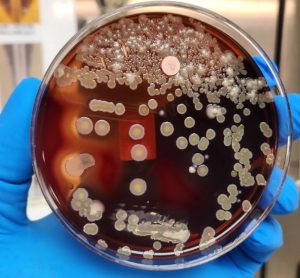
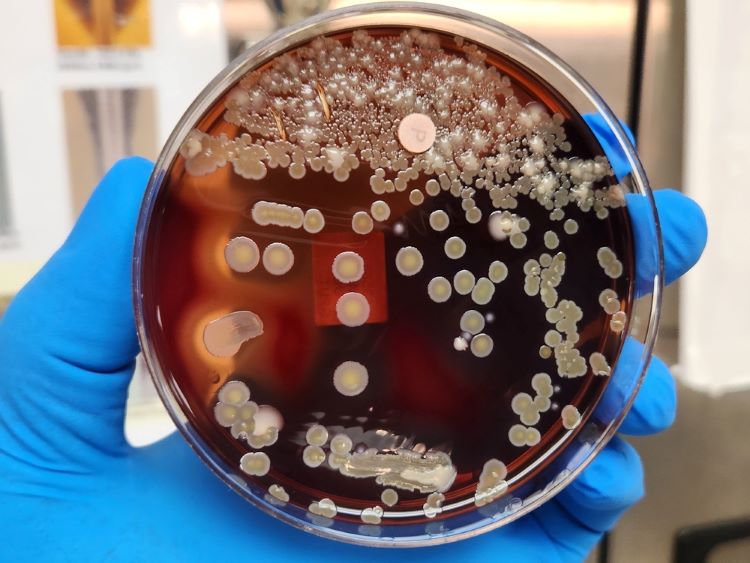
Monitoring pharmaceuticals in the environment
Andreas Häner, an environmental risk assessor at Roche in Group Safety, Security, Health & Environmental protection (SHE), speaks to EPR about how the pharmaceutical industry monitors manufacturing emissions that can impact local environments.