MRI in drug discovery
Posted: 28 February 2012 | | No comments yet
MRI is widely used for clinical diagnosis as well as in research areas such as preclinical drug discovery, clinical development and also in therapy monitoring. MRI allows non-invasive acquisition of tomographic images of soft tissue with high resolution and contrast. Furthermore, its ability to assess organ function in a broad sense renders this technique to a versatile tool to answer specific scientific questions such as drug actions in disease models. Imaging of patho-physiological mechanisms and molecular processes are primarily in the focus of MRI in drug research. Finally, MRI is translational and has thus the potential to bridge the gap between preclinical research on one hand and clinical development or therapy monitoring on the other.
The magnetic moment of some atomic nuclei and with this, nuclear magnetic resonance (NMR), the basis of Magnetic Resonance Imaging (MRI), was predicted in the 1920s by Wolfgang Pauli and successfully demonstrated by Felix Bloch and Edward Purcell in 1946. With the detection of the chemical shift, the technique developed to a powerful analytical method for elucidation of chemical structures. Only decades later, in 1973, Paul Lauterbur published the first image based on NMR signal1. The breakthrough of MRI came with the intro – duction of the gradient based imaging techniques shortly thereafter as developed by Sir Peter Mansfield2. All these persons were later honoured for their contributions to NMR and MRI with the Nobel Prize.
MRI is widely used for clinical diagnosis as well as in research areas such as preclinical drug discovery, clinical development and also in therapy monitoring. MRI allows non-invasive acquisition of tomographic images of soft tissue with high resolution and contrast. Furthermore, its ability to assess organ function in a broad sense renders this technique to a versatile tool to answer specific scientific questions such as drug actions in disease models. Imaging of patho-physiological mechanisms and molecular processes are primarily in the focus of MRI in drug research. Finally, MRI is translational and has thus the potential to bridge the gap between preclinical research on one hand and clinical development or therapy monitoring on the other.The magnetic moment of some atomic nuclei and with this, nuclear magnetic resonance (NMR), the basis of Magnetic Resonance Imaging (MRI), was predicted in the 1920s by Wolfgang Pauli and successfully demonstrated by Felix Bloch and Edward Purcell in 1946. With the detection of the chemical shift, the technique developed to a powerful analytical method for elucidation of chemical structures. Only decades later, in 1973, Paul Lauterbur published the first image based on NMR signal1. The breakthrough of MRI came with the intro - duction of the gradient based imaging techniques shortly thereafter as developed by Sir Peter Mansfield2. All these persons were later honoured for their contributions to NMR and MRI with the Nobel Prize.
MRI is widely used for clinical diagnosis as well as in research areas such as preclinical drug discovery, clinical development and also in therapy monitoring. MRI allows non-invasive acquisition of tomographic images of soft tissue with high resolution and contrast. Furthermore, its ability to assess organ function in a broad sense renders this technique to a versatile tool to answer specific scientific questions such as drug actions in disease models. Imaging of patho-physiological mechanisms and molecular processes are primarily in the focus of MRI in drug research. Finally, MRI is translational and has thus the potential to bridge the gap between preclinical research on one hand and clinical development or therapy monitoring on the other.
The magnetic moment of some atomic nuclei and with this, nuclear magnetic resonance (NMR), the basis of Magnetic Resonance Imaging (MRI), was predicted in the 1920s by Wolfgang Pauli and successfully demonstrated by Felix Bloch and Edward Purcell in 1946. With the detection of the chemical shift, the technique developed to a powerful analytical method for elucidation of chemical structures. Only decades later, in 1973, Paul Lauterbur published the first image based on NMR signal1. The breakthrough of MRI came with the intro – duction of the gradient based imaging techniques shortly thereafter as developed by Sir Peter Mansfield2. All these persons were later honoured for their contributions to NMR and MRI with the Nobel Prize.
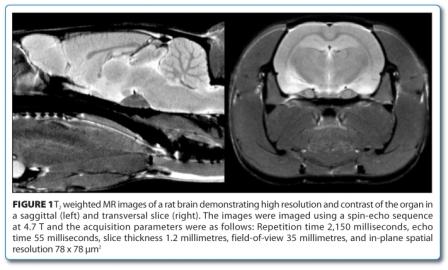
Commercially available MRI scanners were introduced in clinical diagnosis in the 1980’s. The development of the technique and applica – tions in clinical diagnosis was rapid. Today MRI together with CT is considered to be the most important clinical innovation3. The fast acceptance of MRI in clinical practice is based on its versatile features. MRI results in highly resolved images of soft tissue (Figure 1) with high contrast. This contrast can even be optimised for particular applications by adapting the image acquisition parameters. It is non-invasive and thus allows time course studies without side effects. A high number of MRI techniques were developed to not only image organ morphology but also pathophysiological changes and functional processes. Furthermore, combination of imaging with analytical spectroscopy techniques allows insight into the metabolism of an organism. This wealth of non-invasive imaging and spectro – scopy read-outs was also recognised by pharmaceutical research departments and introduced early on.

In drug discovery, MRI is contributing to the profiling of drug candidates by investigating pathological processes in animal disease models and the effect of drugs thereon. Furthermore, MRI, being fully translational, can also contribute to drug development by introducing and validating imaging biomarkers for later clinical trials. Some selected examples shall illustrate the impact that MRI may have in drug develop – ment with emphasis to preclinical research. This review does by far not cover all actual and potential MRI applications in drug research and is driven by personal experience of the author. It should illustrate the major aspects of MRI utilisation in preclinical drug development.
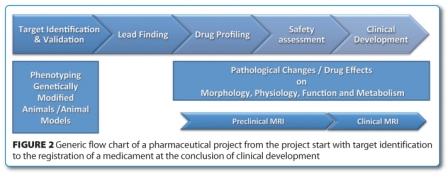
Non-invasive imaging such as MRI can generally be applied to most of the phases of a pharmaceutical project. This starts with target validation and then comprises preclinical drug profiling with respect to efficacy and safety and finally clinical research (Figure 2). Important contributions by MRI can be provided by phenotyping genetically modified animals and characterising conventional animal models at an early state of a project. Later on efficacy and safety of compounds are in the focus. Finally, by translation of preclinical imaging read-outs, MRI may contribute to clinical development and post registration therapy monitoring.

In general, MRI is used to assess the impact of drug treatment on the morphology, structure, metabolism or function of (diseased) organs in tailored animal models or patients. MRI has, however, limitations which have to be considered. It is an insensitive technique. This often limits spatial and/or temporal resolution of images and image series obtained from small laboratory animals. Furthermore, MRI read-outs are similarly to other imaging techniques often indirect with respect to the investigated underlying biological processes and have thus to be thoroughly validated on a case by case basis. Finally, MRI is expensive and sophisticated. Having said this, one has to bear in mind that MRI is in competition with other well established and often less costly non-imaging techniques. Altogether, superiority over alternatives in at least one of the following aspects is a prerequisite for successful MRI applications in drug research: translatability to clinical trials, non-invasiveness allowing time course studies and thus reducing the number of animals per study, and assessment of functional and morphological parameters.
Translational imaging
A straightforward translational read-out appears to be size assessment of tumours in animal models since RECIST (Response Evaluation Criteria In Solid Tumors4) is well established in the clinic to assess anti-tumour treatment effect. However, subcutaneous tumours, often used in preclinical studies, can easily be quantified with a calliper rendering sophisticated in vivo imaging techniques superfluous. Angiogenesis in tumours, however, has received much attention in oncology research and is one of the targets of anti-tumour therapy. The aim of antiangiogenic therapy is to suppress the essential process of blood vessel formation and maturation for growing tumours. Quantification of angiogensis by dynamic contrast enhanced MRI (DCE-MRI) and hence its inhibition by pharmacological treatment is well established in pre-clinical as well as in clinical research5. DCEMRI allows analysing the structure as well as the function of the forming tumour vascular bed non-invasively. For that, it is assessing dynamically the pharmacokinetics of an intravenously injected contrast agent while passing through the tumour vasculature6. This technique is meanwhile a well-accepted biomarker for drug efficacy of anti-angiogenic or vascular disrupting agents also in many clinical trials. A prerequisite for that was the successful demonstration of the translatability of DCE-MRI from the preclinical arena to clinical setting which was performed for a particular vascular endothelial growth factor (VEGF) receptor inhibitor7. A review article from O’Connor et al summarises the important impact this technique as to many clinical trials8.
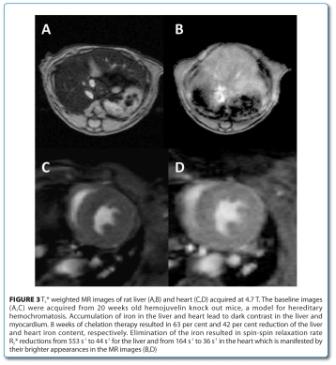
Iron overload in the liver and heart is a lifethreatening condition for patients receiving large numbers of blood transfusions due to diseases such as thalassaemia. Excess iron can nowadays be eliminated pharmacologically from the organs by chelation therapy. With this respect, monitoring of organ iron content for assessing drug efficacy for monitoring therapy or in clinical studies of novel chelators is crucial. MRI can perform this non-invasively and offers thus advantages over its alternatives such as serum ferritin assessment which does not correlate well with organ iron load or organ biopsy which, due its invasiveness, cannot be practiced repeatedly. Organ iron assessment by MRI is based on a positive correlation of iron content with tissue water proton transversal relaxation rates R2 or R2*9,10. This stems from the facts that iron stores express ferromagnetic properties. Quantitative R2* imaging was successfully applied preclinically to monitor the effect of an orally bioavailable iron chelator in a model of hereditary hemochromatosis11. Figure 3 shows dark appearances iron over – loaded mouse liver and heart as compared to the same organs after successful chelation therapy. Hence non-invasive organ iron assessment by MRI is another example of a translational and well-established technique12.

Time course studies
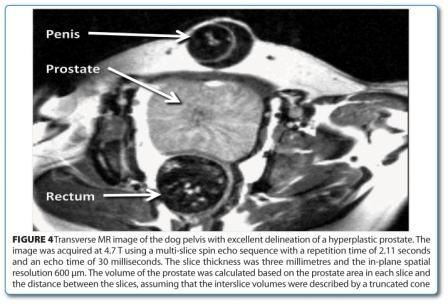
A strong argument in favour of MRI is its non-invasive nature allowing time course (longitudinal) studies. Firstly, each animal serves as its own control in this type of studies and with this strengthens statistical analysis. Furthermore, the number of animals to be included in a time course study can be minimised since none have to be sacrificed at intermediate time points. Two early examples from our lab are mentioned here. Besides humans, prostate hyperplasia occurs in very few animal species. One of these is the dog, which thus represents an ideal animal model for drug characterisation. Dogs, on the other hand, can only be utilised in very small numbers for pharmacological studies and noninvasive read outs are thus predestined. So the effect of 5α reductase inhibitor treatment of prostate hyperplasia in dogs13 could be successfully monitored during a 12-weektreatment and an eight week wash-out period. Figure 4 shows the excellent delineation of a hyperplasic prostate in a T2-weighted MR image. Another example consists in spleen and inguinal lymph node size assessment as a measure for murine leukaemia virus infection progression in mice for a time period of more than 80 days14. With this, the progression of the disease could easily be followed and statistically relevant results could be obtained requiring the use of only a few animals.

Multimodal imaging of pathophysiological events
The full potential of MRI in drug research lays in the non-invasive investigation of pathophysiological events and the effect of drug treatment thereon. The comprehensive assessment of patho-physiological cascade of cerebral stroke with various MRI techniques is described in Figure 515,16.
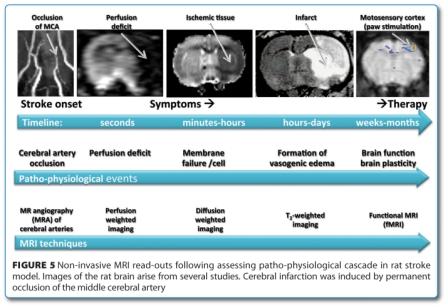

Cerebral ischemia is induced by occlusion of the middle cerebral artery (MCAO) which is immediately visualised by MR angiography (MRA). The threedimensional time-of-flight (TOF) technique visualises non-invasively the cerebral vasculature of the rat without the use of contrast agent17. It is applicable to investigate therapeutic interventions such as thrombolytic treatments. The MCAO results in a massive ischemic area as seen in the perfusion weighted image. This technique is based on the dynamic assessment of the first passage of a contrast agent bolus18 and can equally be used in preclinical and clinical settings. Within minutes, ion homeostasis is lost in the ischemic area due to failure of energy metabolism resulting in cell swelling. This event can be visualised by diffusion weighted imaging (DWI)19, a technique assessing tissue water diffusion rate. The ischemic lesion can be seen by its low apparent diffusion coefficient i.e. characterised by a dark contrast on the MR images. The full blown infarct is characterised by massive vasogenic edema, which can in turn be easily seen on T2 weighted images. Infarct size as measured with MRI was intensively used to assess the effect of neuroprotective drugs in preclinical research20-22. Finally, functional MRI (fMRI) can assess brain function loss due to cerebral ischemia and later on the spontaneous or therapy- induced restoration of function23. fMRI in preclincial research is, however, restricted to a few stimulation paradigms due to the need for anesthesia of the animals during data acquisition to a few stimulation paradigms. fMRI on awake and trained animals like monkeys24 or rats25 are, nevertheless, feasible.
Molecular and cellular imaging
Molecular imaging emerged as a discipline combining molecular biology and in vivo imaging. Due to the need for very high sensitivity in order to observe sparse molecular events optical or radiological imaging techniques are often used. However, these imaging modalities are hampered by their low spatial resolution. In some cases the molecular events can, however, be amplified with specific contrast agents such that MRI can visualise them with high precision. Usually iron oxide containing contrast agents are used which result in a hypointense contrast. The monitoring of the fate of injected iron-oxide-labelled stem cells in the body is an example for this approach. It could be shown that the stem cells migrate to the lesion after cerebral ischemia in the rat and may cause functional recovery23. In order to visualise single cells those were extracted and loaded in vitro with contrast agent prior to their injection. For assessing macrophage invasion, a different labelling approach is chosen. Here the contrast agent loading is performed in the animal without the necessity to extract the cells.
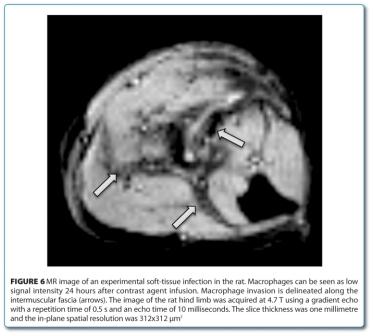

Figure 6 shows macrophage invasion after intramuscular bacterial infection26. Furthermore, the involvement of macrophages in cerebral ischemia27, or multiple sclerosis models28 could be demonstrated in vivo. Also, gene expression could successfully be visualised with MRI techniques. By using an enzymatically activa – table contrast agent with β-galactosidase the expression of this transfected gene could be demonstrated29. The contrast agent was blocked with a β-galactosidase cleavable side chain which developed its activity upon its elimination. A further example consists of the expression of an engineered transferrin receptor resulting in the uptake of an iron containing contrast agent30. With this, the magnetically labelled cells can be visualised in vivo by MRI.
Phenotyping of genetically modified animals is an often used approach to elucidate underlying molecular causes for diseases. Here MRI may play a role in assessing downstream effects of the molecular mechanisms. Such an example is the elucidation of signalling mechanisms by which obesity leads to insulin resistance31. It could be shown that S6 Kinase 1 (S6K1) deficient mice had enhanced insulin sensitivity. At the same time, these mice were protected against age- and diet-induced obesity as shown in vivo during several months of high fat diet by MRI. This result is embedded into ex vivo molecular and histological read-outs and altogether leads to a better understanding of the S6K1 signalling function in obesity and insulin resistance.
Conclusion
The above described examples give just a tiny glimpse to the wealth of potential MRI applications in preclinical research. MRI, when used appropriately, can answer important biological questions in the context of many projects. Furthermore, MRI by its translational nature can also link preclinical and clinical research. Altogether, it has the potential to contribute substantially to the drug finding process in pharmaceutical industry.
Acknowledgment
The author thanks Nicolay Beckmann and Michael Smith for carefully reading the manuscript and valuable discussions.
About the author
Dr. Peter Allegrini has a diploma in Chemistry (1977) and a PhD in Biochemistry (1981) from the University of Berne, Switzerland. Peter then joined Professor J. Seelig’s lab at the Biocenter in Basel where he investigated biological membranes using solid phase NMR. He then took over the task to build up an in vivo MRI/MRS lab at Ciba-Geigy AG (now Novartis AG). In his function as head of MRI lab, he was collaborating with research teams in the fields of cerebral stroke, cardiovascular diseases, endocrinology, infectious diseases, obesity, oncology, iron overload and musculoskeletal diseases.
References
1. Lauterbur PC. Image Formation by Induced Local Interactions: Examples Employing Nuclear Magnetic Resonance. Nature 1973;242:190-191
2. Mansfield P, Maudsley AA. Line scan proton spin imaging in biological structures by NMR. Physics in Medicine & Biology 1976;21(5):847-852
3. Fuchs VR, Sox HC. Physicians’ views of the relative importance of thirty medical innovations. Health Affairs 2001;20(5):30-42
4. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228-247
5. Padhani AR, Leach MO. Antivascular cancer treatments: functional assessments by dynamic contrast-enhanced magnetic resonance imaging. [Review] [146 refs]. Abdominal Imaging 2005;30(3):324-341
6. Weidensteiner C, Rausch M, McSheehy PMJ, Allegrini PR. Quantitative dynamic contrast-enhanced MRI in tumor-bearing rats and mice with inversion recovery TrueFISP and two contrast agents at 4.7 T. JMRI 2006;24(3):646-656
7. Lee L, Sharma S, Morgan B, Allegrini P, Schnell C, Brueggen J, Cozens R, Horsfield M, Guenther C, Steward WP, Drevs J, Lebwohl D, Wood J, McSheehy PM. Biomarkers for assessment of pharmacologic activity for a vascular endothelial growth factor (VEGF) receptor inhibitor, PTK787/ZK 222584 (PTK/ZK): translation of biological activity in a mouse melanoma metastasis model to phase I studies in patients with advanced colorectal cancer with liver metastases. Cancer Chemotherapy & Pharmacology 2006;57(6):761-771
8. O’connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. [Review] [42 refs]. British Journal of Cancer 2007;96(2):189-195
9. St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 2005;105(2):855-861
10. Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, Li CS, Wang WC, Ware RE, Hillenbrand CM. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 2009;113(20):4853-4855
11. Nick HP, Allegrini PR, Fozard L, Junker U, Rojkjaer L, Salie R, Niederkofler V, O’Reilly T. Deferasirox Reduces Iron Overload in a Murine Model of Juvenile Hemochromatosis. Exp Biol Med 2009;234(5):492-503
12. Porter JB, Davis BA. Monitoring chelation therapy to achieve optimal outcome in the treatment of thalassaemia. Best Practice & Research Clinical Haematology 2002;15(2):329-368
13. Hausler A, Allegrini PR, Biollaz M, Batzl C, Scheidegger E, Bhatnagar AS. Cgp 53153 – a new potent inhibitor of 5- alpha-reductase. Journal of Steroid Biochemistry & Molecular Biology 1996;57(3-4):187-195
14. Allegrini PR, Wachsmuth ED. Course of murine leukemia retrovirus infection determined in vivo by magnetic resonance imaging. Laboratory Investigation 1990;63(4):568-575
15. Weber R, Ramos-Cabrer P, Hoehn M. Present status of magnetic resonance imaging and spectroscopy in animal stroke models. [Review] [101 refs]. Journal of Cerebral Blood Flow & Metabolism 2006;26(5):591-604
16. Burgess RE, Kidwell CS. Use of MRI in the assessment of patients with stroke. [Review]. Current Neurology & Neuroscience Reports 2011;11(1):28-34
17. Reese T, Bochelen D, Sauter A, Beckmann N, Rudin M. Magentic resonance angiography of the rat cerebrovascular system without the use of contrast agent. NMR Biomed 1999;12:189-196
18. Calamante F. Perfusion MRI using dynamicsusceptibility contrast MRI: quantification issues in patient studies. [Review]. Topics in Magnetic Resonance Imaging 2010;21(2):75-85
19. Hoehn-Berlage M, Eis M, Back T, Kohno K, Yamashita K. Changes of Relaxation Times (T1, T2) and Apparent Diffusion Coefficient After Permanent Middle Cerebral Artery Occlusion in the Rat: Temporal Evolution, Regional Extent, and Comparison with Histology. Magn Reson Med 1995;34:824-834
20. Sauer D, Weber E, Luond G, Dasilva F, Allegrini PR. The competitive nmda antagonist cgp 40116 permanently reduces brain damage after middle cerebral artery occlusion in rats. Journal of Cerebral Blood Flow & Metabolism 1995;15(4):602-610
21. Wiessner C, Sauer D, Alaimo D, Allegrini PR. Protective effect of a caspase inhibitor in models for cerebral ischemia in vitro and in vivo. Cellular & Molecular Biology 2000;46(1):53-62
22. Wiessner C, Bareyre FM, Allegrini PR, Mir AK, Frentzel S, Zurini M, Schnell L, Oertle T, Schwab ME. Anti-Nogo-A antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. Journal of Cerebral Blood Flow & Metabolism 2003;23(2):154-165
23. Ramos-Cabrer P, Justicia C, Wiedermann D, Hoehn M. Stem cell mediation of functional recovery after stroke in the rat. PLoS ONE [Electronic Resource] 2010;5(9):e12779, 2010
24. Schmid MC, Oeltermann A, Juchem C, Logothetis NK, Smirnakis SM. Simultaneous EEG and fMRI in the macaque monkey at 4.7 Tesla. Magn Reson Imaging 2006;24(4):335-342
25. Huang W, Heffernan ME, Li Z, Zhang N, Overstreet DH, King JA. Fear induced neuronal alterations in a genetic model of depression: an fMRI study on awake animals. Neurosci Lett 2011;489(2):74-78
26. Kaim AH, Jundt G, Wischer T, O’Reilly T, Frohlich J, von Schulthess GK, Allegrini PR. Functional-morphologic MR imaging with ultrasmall superparamagnetic particles of iron oxide in acute and chronic soft-tissue infection: study in rats. Radiology 2003;227(1):169-174
27. Rausch M, Baumann D, Neubacher U, Rudin M. In-vivo visualization of phagocytotic cells in rat brains after transient ischemia by USPIO. NMR Biomed 2002;15(4):278-283
28. Rausch M, Hiestand P, Baumann D, Cannet C, Rudin M. MRI-based monitoring of inflammation and tissue damage in acute and chronic relapsing EAE. Magnetic Resonance in Medicine 50(2):309-14, 2003;50(2):309- 314
29. Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. Nature Biotechnology 2000;18(3):321-325
30. Weissleder R, Moore A, Mahmood U, Bhorade R, Benveniste H, Chiocca EA, Basilion JP. In vivo magnetic resonance imaging of transgene expression. Nature Medicine 2000;6(3):351-355
31. Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 Protects Against Age and Diet-Induced Obesity While Enhancing Insulin Sensitivity. Nature 2004;431(7005):200-205
Issue
Related topics
Drug Discovery, Magnetic Resonance Imaging (MRI), Preclinical Research




