Breaking old habits: Moving away from commonly used buffers in pharmaceuticals
Posted: 10 July 2012 | | No comments yet
One of the key factors in stabilising proteins is determining the optimal pH and buffer system to provide adequate solubility and stability. Currently, three buffers, citrate, phosphate and acetate, make up the majority of buffers used in parenteral pharmaceuticals approved by the FDA, but less precedented excipients are certainly available to use in commercial dosage forms. A number of alternative buffers have also gone through the approval process to be proven safe, and the use of histidine and tromethamine is becoming increasingly common in parenteral formulations. This article highlights the advantages of some lesser known buffers with the hope that bringing these excipients into the clinic and market more often will help to augment the tools formulators have available in achieving stable protein formulations.
The US Food, Drug and Cosmetic Act of 1938 required manufacturers and pharmaceuticals companies to be responsible for the safety of drug additives / excipients in their products in response to a tragedy where 100 children were killed from the presence of diethylene glycol in an antibacterial product. To help clarify what is needed to establish a new excipient, the FDA released a guidance document in 2005 entitled ‘Nonclinical Studies for the Safety Evaluation of Pharmaceutical Excipients’, outlining the safety studies needed in order to have an excipient approved1,2. Recent articles have called for the need for more alternative excipients for the use in formulations1,3,4. Formulators generally prefer precedented excipients because of their well-established safety profile as well as the risks and costs associated with approving a new excipient3. When qualifying a new excipient, safety studies need to be performed and in the case of an adverse event, doubt exists on whether the adverse event was due to the excipient or the product. When using an excipient, while previous experience with any injectable route of administration is valuable in the justification of its use, it is worth noting that under current regulations changing the route of administration or increasing the concentration beyond its current highest dose may require additional safety data1,2. The need for new excipients is well recognised, but expanding our knowledge in the use of less widely used excipients is just as important while offering an easier regulatory path forward.
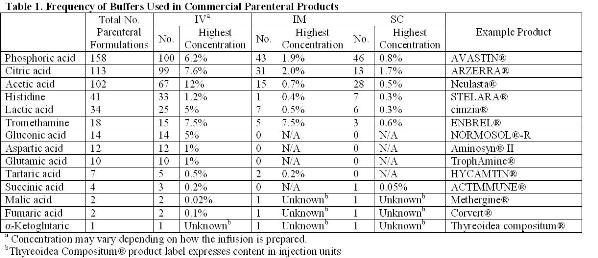
Determining the correct pH and buffer system is arguably the most critical step in formulating a protein to ensure its solubility and stability (chemical and physical)3,5-7. The article will focus on the stabilisation of biotherapeutics. Buffers found in both small molecules and protein parenteral formulations approved by the FDA will be referenced as justification for exploring the stabilising effects of these buffers on proteins. Table 1 lists the number of unique formulations found searching the labels of products approved by the FDA. Identical formulations arising from the formulation of generic products or different dosage strengths were excluded. Whenever the salt form of the buffer is alluded to, the buffer should be assumed to be titrated with sodium hydroxide or hydrochloric acid as these are the most common titrants.

The most common buffers found to be used in parenteral formulations have been citrate, phosphate and acetate. Some of the strengths and weaknesses associated with these excipients will be highlighted. The goal of this paper is to draw attention to other approved, but less commonly used, buffering agents that require more extensive use to become generally accepted. While this article will exclusively focus on buffers, the same argument extends to other types of excipients. As biotechnology evolves as an industry and more unique protein scaffolds are developed, it will become increasingly important to find new ways of stabilising these proteins. Sometimes the most common excipients will not be sufficient to provide adequate stability or enable drug delivery technologies.
Commonly used buffers
Sodium phosphate
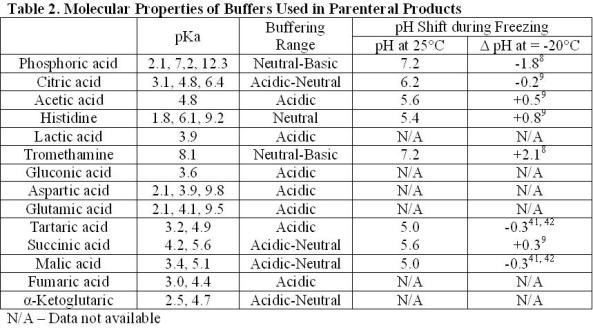
Sodium phosphate (pKa 2.1, 7.2, and 12.3) is the most commonly used buffer found in parenteral formulations (Table 1). The most notable limitation in using phosphate as a buffer is the effect of freezing on changes in pH. The shift in pH during freezing has been studied extensively. The pH shift is attributed to the selective crystallisation of the dibasic salt. Crystallisation can be affected by the rate of freezing, starting pH and the presence of other cosolutes. A shift in pH of approximately two units is fairly typical during freezing, but shifts in pH up to 3.6 units have been reported8-10. The changing pH environment has the potential to destabilise protein during processing of bulk drug substance or lyophilisation of drug product solutions11-17. This is most concerning under conditions where high phosphate concentrations are used or other crystallising excipients are present as these conditions help to induce phosphate crystallisation. Solutions containing high concentrations of protein or non-crystallisable solutes (e.g. sucrose) will inhibit phosphate crystallisation and minimise pH shifts.
Citric acid
Citric acid is one of the most commonly used buffers (Table 1). It is a trivalent buffer containing three carboxylic acids with pKa of 3.1, 4.8 and 6.4 (Table 2) offering a wide buffering range5. According to the FDA’s Inactive Ingredient Database, citrate is found in over 100 approved, injectable products, giving it a large history of use and proving its safety.

However, studies comparing subcutaneous injections of citrate-buffered formulations against com parable formulations buffered with phosphate or histidine have established that citratebuffered solutions induced more pain upon subcutaneous injection18-20. While this pain was relatively short in duration (less than two minutes), it raises concerns of patient compliance with self-administering injections containing citrate18. When formulating with citric acid, the target product profile and intended route of administration should be noted. When frequent subcutaneous injections are intended, another buffering agent may be more appropriate.
Acetic acid
Acetic acid has a pKa of about 4.8, offering a buffering range of 3.7-5.6 (Table 2). The use of acetic acid is ideal for the formulation of proteins stable in the liquid state under acidic conditions. The lyophilisation of acetic acid solutions is accompanied by an increase in pH due to the sublimation of the volatile acid form of the buffer, leaving behind the basic salt. This is potentially destabilising to the protein and makes control of the pH in the lyophilised drug products difficult.
Additional approved buffers
Tromethamine
Tromethamine (Tris) (pKa 8.1) has similar properties to phosphate (pKa 7.2) and could be used as a substitute near neutral pH conditions. Both buffers exhibit a pH change during freezing. Under identical conditions, the pH shift for tromethamine (+2.1) was about equal to that of phosphate ( 1.8), but in the opposite direction (Table 2)8.
Choosing one buffer over another would then likely be dependent on the sensitivity of the individual protein to the direction of the pH shift and any specific protein-buffer interactions. Tromethamine and histidine have been shown to be better stabilisers than phosphate when erythropoietin was subjected to high temperature stress, preventing aggregation by aiding the reversibility of unfolding21. Ure2p was shown to be more stabile against fibril formation when formulated in tromethamine versus phosphate22.
Histidine
Histidine, an essential amino acid, is becoming increasingly common in formulations of protein therapeutics. Having a pKa of 6.1, it is ideal for the protein formulations near neutral conditions (Table 2).
Histidine has been shown to protect a monoclonal antibody in both the liquid and lyophilised state against heat stress23-25. Studies examining the stability of interferon-tau exhibited that formulations containing histidine (followed by Tris then phosphate) were the most stable against heat-induced aggregation. This stabilisation was attributed to direct binding of histidine to the protein26. During lyophilisation, histidine and aspartic acid have been shown to protect antibodies by acting as a hydrogen bond donor to preserve intramolecular β-sheets24-25. Additionally, histidine has been found to have the properties of an antioxidant because of its ability to bind iron ions and singlet oxygen24,27. The drawback of using histidine is that histidine solutions have been observed to change colour under accelerated temperatures and acidic pH as well as extract iron from stainless steel24.
Gluconic, lactic and tartaric acid
Gluconic, lactic acid and tartaric acid are excipients that have almost exclusively been used in small molecule formulations. Calcium gluconate has been licensed for the treatment of hydrogen fluoride burns through a topical application. In more severe cases an infusion of a four per cent calcium gluconate solution in saline has been used for prevention of tissue damage28. Out of the 23 commercially available, injectable formulations containing lactic acid found in the FDA Inactive Ingredient database, four formulations are used for biologics – two peptides and two proteins. Of the 12 formulations containing tartaric acid, only one has been used in formulating a protein. These buffers may be suitable substitutes for acetate and citrate.
Gluconic acid is a compound naturally formed from glucose oxidation. It is an interesting compound as it has the potential to act as a buffer, stabiliser and chelating agent due to it having a structure containing carboxylic acids and hydroxyl groups.
Tartarate has been shown to improve the retention of monomer over citrate in an antibody formulation at pH 4.0-4.5 when stored as a liquid at 25°C29. It has been hypothesised that the hydroxyl groups in tartarate, citrate, malate and by extension lactate and gluconate could supply stabilising hydrogen bonds in the amorphous phase of a lyophilised cake30-31.
Aspartic and glutamic acid
Both aspartic and glutamic acid are found in infusions given to infants and pediatric patients with insufficient plasma concentrations of amino acids. In TrophAmine®, aspartate and glutamate are infused at concentrations of 22 mM and 34 mM, respectively32. These concentrations are sufficiently high enough to provide adequate buffering of protein formulations.
Glutamic acid when formulated with equal amounts of arginine has been shown to improve the stability and solubility of intra-cellular proteins23,33 as well as prevent aggregation during reconstitution23,34-35. Studies on insulin and human serum albumin indicate that the addition of glutamate and aspartate were able to inhibit the formation of aggregates36-38. Studies with pegfilgrastim indicated the stability of pegfilgrastim formulated in glutamate or formate was comparable to acetate39.
Citric acid cycle intermediates
Citrate, fumarate, α-ketoglutarate, malate and succinate are all intermediates in the citric acid cycle found to be safe and effective as buffers or counter ions in injectable formulations. Fumarate, α-ketoglutarate, malate and succinate have been used in a limited number of products (Table 1). It stands to reason that the other intermediates of the Krebs cycle may also be appropriate for use in parenterals. These compounds include aconitate, isocitrate and oxaloacetate. Being divalent and trivalent carboxylic acids, they have pKa’s ranging from 2.0 to 6.4, making these compounds suitable for a wide range of formulations (Table 2).
Due to the limited use of these compounds as buffers, it is unclear whether the same pain on injection associated with subcutaneous injections of citrate would be associated with these compounds. However, there are other advantages associated with their use. Citrate has been shown to induce gelation of antibody solutions when the protein concentration was above 100 mg/mL. This was attributed to the trivalent nature of the buffer. Succinate, having only two carboxylic acids, was not found to have the same effect at the same pH40.
Conclusion
Determining the correct pH and buffer system is critical for bringing biotherapeutics into the clinic and to the market. The need for new excipients as well as a better understanding of existing excipients is highly desirable. Histidine, tromethamine and a number of other alternative buffers offer advantages over conventionally used excipients. The use of these alternative buffers can do nothing but help to augment the tools formulators have and may enable the development of a therapeutic that otherwise may have failed. To stabilise the vast number of protein therapeutics in development, all approaches should be open for consideration.
References
1. Osterberg, R. E., Demerlis, C. C., Hobson, D. W., Mcgovern, T. J. (2011) Trends in Excipient Safety Evaluation. Int. J. Tox. 30, 600-610
2. FDA (2005) Guidance for Industry: Nonclinical Studies for the Safety Evaluation of Pharmaceutical Excipients
3. Jorgensen, L., Hostrup, S., Moeller, E. H., Grohganz H. (2009) Recent Trends in Stabilising Peptides and Proteins in Pharmaceutical Formulation – Considerations in the Choice of Excipients. Expert Opinion. 6, 1219-1230
4. Kamerzell, T. J., Esfandiary, R., Joshi, S. B., Middaugh, C. R., Volkin, D. B. (2011) Protein–Excipient Interactions: Mechanisms and Biophysical Characterization Applied to Protein Formulation Development. Adv. Drug Delivery Reviews. 63, 1118–1159
5. Stoll, V. S., Blanchard, J. S. (1990) Buffers: Principles and Practice. Methods in Enzymology. 182, 24-38
6. Trevino, S. R., Scholtz, J. M., Pace, C. N. (2008) Measuring and Increasing Protein Solubility. J. Pharm. Sci. 97, 4155-4166
7. Wang, W., Nema, S., Teagarden, D. (2010) Protein Aggregation-Pathways and Influencing Factors. Int. J. Pharmaceutics. 390, 89–99
8. Williams-Smith, D. (1977) Changes in Apparent pH on Freezing Aqueous Buffer Solutions and their Relevance to Biochemical Electron-Paramagnetic-Resonance Spectroscopy. Biochem. J. 167, 593-600.
9. Kolhe, P., Amend, E., Singh, S. K. (2010) Impact of Freezing on pH of Buffered Solutions and Consequences for Monoclonal Antibody Aggregation. Biotechnol. Prog. 26, 727-733
10. Gomez, G., Pikal, M. J., Rodriguez-Hornedo, N. (2001) Effect of Initial Buffer Composition on pH Changes during Far-From-Equilibrium Freezing of Sodium Phosphate Buffer Solutions. Pharm. Res. 18, 90-97
11. Hatley R.H.M., Franks, F. (1989) The Cold-Induced Denaturation of Lactate Dehydrogenase at Sub-Zero Temperatures in the Absence of Perturbants. FEBS Lett 257,171-173
12. Almarsson, O., Seburg, R.A., Godshall, D., Tsai, E. W., Kaufman, M. J. (2000), Solid-State Chemistry of a Novel Carbapenem with a Releasable Sidechain, Tetrahedron 56, 6877–6885
13. Lam, X. M., Costantino, H. R., Overcashier, D. E., Nguyen, T. H., and Hsu, C. C. (1996) Replacing Succinate with Glycolate Buffer Improves the Stability of Lyophilized Interferongamma. Int. J. Pharm. 142, 85–95
14. Bell, L.N., Labuza, T.P. (1991) Aspartame Degradation Kinetics as Affected by pH in Intermediate and Low Moisture Food Systems, J. Food Sci. 56, 17–20
15. Sarciaux, J.M., Mansour, S., Hageman, M.J., Nail, S.L. (1999) Effects of Buffer Composition and Processing Conditions on Aggregation of Bovine IgG during Freeze-Drying, J. Pharm. Sci. 88, 1354–1361
16. Pikal-Cleland, K.A., Carpenter, J.F. (2001) Lyophilization- Induced Protein Denaturation in Phosphate Buffer Systems: Monomeric and Tetrameric b-galactosidase, J. Pharm. Sci. 90, 1255–1268
17. Shalaev, E.Y., Gatlin, L.A. (2010) The Impact of Buffer on Solid-State Properties and Stability of Freeze-Dried Dosage Forms, in Formulation and Process Development Strategies for Manufacturing Biopharmaceuticals (eds F. Jameel and S. Hershenson), John Wiley & Sons, Inc., Hoboken, NJ, USA
18. Laursen, T., Hansen, B., Fisker, S. (2006) Pain Perception After Subcutaneous Injections of Media Containing Different Buffers. Basic & Clinical Pharmacology & Toxicology. 98, 218–221
19. Veys, N., Dhondt, A., Lameire, N., (1998) Pain at the Injection Site of Subcutaneously Administered Erythropoietin: Phosphate-Buffered Epoetin Alpha Compared to Citrate-Buffered Epoetin Alpha and Epoetin Beta. Clin. Nephrol. 49, 41–44
20. Yu, A. W., Leung, C. B. , Li, P. K., Lui, S. F., Lai, K. N. (1998) Pain Perception Following Subcutaneous Injections of Citrate-Buffered and Phosphate-Buffered Epoetin Alpha. Int. J. Artif. Organs. 21, 341–343
21. Arakawa, T., Philo, J.S., Kita, Y. (2001) Kinetic and Thermodynamic Analysis of Thermal Unfolding of Recombinant Erythropoietin. Biosci. Biotechnol. Biochem. 65, 1321-1327
22. Zhu, L., Zhang, X.J., Wang, L. Y., Zhou, J. M., Perrett, S. (2003) Relationship between Stability of Folding Intermediates and Amyloid Formation for the Yeast Prion Ure2p: A Quantitative Analysis of the Effects of pH and Buffer System. J. Mol. Biol. 328, 235–254
23. Arakawa, T. Tsumoto, K., Kita, Y., Chang, B., Ejima D. (2007) Biotechnology Applications of Amino Acids in Protein Purification and Formulations. Amino Acids. 33, 587–605
24. Chen, B., Bautista, R., Yu, K., Zapata, G. A., Mulkerrin, M. G., Chamow, S. M. (2003) Influence of Histidine on the Stability and Physical Properties of a Fully Human Antibody in Aqueous and Solid Forms. Pharmacol. Res. 20, 1952–1960
25. Tian, F., Middaugh, C. R., Offerdahl, T., Munsona, E., Sane, S., Rytting, J. H. (2007) Spectroscopic Evaluation of the Stabilization of Humanized Monoclonal Antibodies in Amino Acid Formulations. Int. J. Pharmaceutics. 335, 20–31
26. Katayama, D. S., Nayar, R., Chou, D. K., Valente, J. J., Cooper, J., Henry, C. S., Vander Velde, D. G., Villarete, L., Liu, C. P., Manning, M.C. (2006) Effect of Buffer Species on the Thermally Induced Aggregation of Interferon- Tau. J. Pharm. Sci. 95, 1212-1226
27. Wade, A. M., Tucker, H.N. (1998) Antioxidant Characteristics of L-Histidine. J. Nutr. Biochem. 9, 308-315
28. Thomas, D., Jaeger, U., Sagoschen, I., Lamberti, C., Wilhelm, K. (2009) Intra-Arterial Calcium Gluconate Treatment after Hydrofluoric Acid Burn of the Hand. Cardiovasc. Intervent. Radiol. 32, 155–158
29. Zheng, J. Y., Janis, L. J., (2006) Influence of pH, Buffer Species, and Storage Temperature on Physicochemical Stability of a Humanized Monoclonal Antibody LA298. Int. J. Pharmaceutics. 308, 46 51
30. Izutsu, K. I., Kadoya, S., Yomota, C., Kawanishi, T., Yonemochi, E., Terada, K. (2009) Freeze-Drying of Proteins in Glass Solids Formed by Basic Amino Acids and Dicarboxylic Acids. Chem. Pharm. Bull. 57, 43-48
31. Izutsu, K. I., Kadoya, S., Yomota, C., Kawanishi, T., Yonemochi, E., Terada, K. (2009) Stabilization of Protein Structure in Freeze-Dried Amorphous Organic Acid Buffer Salts. Chem. Pharm. Bull. 57, 1231-1236
32. B. Braun Medical Inc. TROPHAMINE – isoleucine, leucine, lysine acetate, methionine, phenylalanine, threonine, tryptophan, valine, cysteine hydrochloride, histidine, tyrosine, n-acetyl-tyrosine, alanine, arginine, proline, serine, glycine, aspartic acid, glutamic acid and taurine solution
33. Golovanov A.P., Hautbergue G.M., Wilson S.A., Lian L.Y. (2004) Simple Method for Improving Protein Solubility and Long-Term Stability. J. Am. Chem. Soc. 126, 8933–8939
34. Zhang M.Z., Wen J., Arakawa T., Prestrelski S.J. (1995) A New Strategy for Enhancing the Stability of Lyophilized Protein: The Effect of the Reconstitution Medium on Keratinocyte Growth Factor. Pharmacol. Res. 12, 1447–1452
35. Zhang M.Z., Pikal K., Nguyen T., Arakawa T., Prestrelski S.J. (1996) The Effect of the Reconstitution Medium on Aggregation of Lyophilized Recombinant Interleukin-2 and Ribonuclease A. Pharmacol. Res. 13: 643–646
36. Quinn, R., Andrade, J. D. (1983) Minimizing the Aggregation of Neutral Insulin Solutions. J. Pharm. Sci. 72, 1472-1473
37. Bringer, J., Heldt, A., Grodsky, G.M. (1981) Prevention of Insulin Aggregation by Dicarboxylic Amino Acids during Prolonged Infusion. Diabetes, 30, 83-85
38. Saso, L., Valentini, G., Casini, M.L., Matti, E., Braghiroli, L., Mazzanti, G., Panzironi, C., Grippa, E., Silvestrini, B. (1999) Inhibition of Protein Denaturation by Fatty Acids, Bile Salts and Other Natural Substances: A New Hypothesis for the Mechanism of Action of Fish Oil in Rheumatic Diseases. Jpn. J. Pharmacol.79, 89-99
39. Piedmonte, D. M., Treuheit, M. J. (2008) Formulation of Neulasta (pegfilgrastim). Adv. Drug Delivery Reviews. 60, 50–58
40. Esue, O., Kanai, S., Liu, J., Patapoff, T. W. Shire, S. J., (2009) Carboxylate-Dependent Gelation of a Monoclonal Antibody. Pharm. Res. 26, 2478-2485
41. Sundaramurthi, P., Suryanarayanan, R., (2011) Predicting the Crystallization Propensity of Carboxylic Acid Buffers in Frozen Systems—Relevance to Freeze- Drying. J. Pharm. Sci. 100, 1288–1293
42. Sundaramurthi, P., Suryanarayanan, R., (2011) Thermophysical Properties of Carboxylic and Amino Acid Buffers at Subzero Temperatures: Relevance to Frozen State Stabilization. J. Phys. Chem. B. 115, 7154–7164
About the author
David Sek earned his BS in Chemistry from Bates College and his MS in Chemistry and Chemical Biology from Northeastern University. He has 10 years of experience as a formulation scientist and has spent the past eight years working at Pfizer studying the stability of novel biotherapeutics.
Issue
Related topics
Drug Development, Excipients, Lyophilisation, protein structure