Rapid micro methods and the next generation in ATP bioluminescence
Posted: 25 October 2012 |
This is the fifth paper in our continuing series on Rapid Microbiological Methods (RMM) that will appear in European Pharmaceutical Review during 2012. As many of you know, I am always on the lookout for the next generation of rapid microbiological method (RMM) technologies and solutions. In this article, I have invited Noe Miyashita, a researcher from Hitachi Plant Technologies, to describe a novel ATP bioluminescence technology platform that she and her colleagues are currently working on. But in order to frame this discussion, it is appropriate to provide some background material on the fundamental basics of ATP bioluminescent methods.
ATP bioluminescence is the generation of light by a biological process, and is most recognised in the tails of the American firefly Photinus pyralis. First discovered in 1947 by William McElroy, he described the ATP bioluminescence reaction in which ATP (Adenosine Triphosphate) is enzymatically consumed to produce light. Specifically, in the presence of the substrate luciferin, the enzyme luciferase will use the energy from ATP to oxidise luciferin and release photons (light at a wavelength of 562 nanometres). The photons can then be detected and measured by a luminometer equipped with a photomultiplier tube. Figure 1 provides an illustration of this chemical reaction.

This is the fifth paper in our continuing series on Rapid Microbiological Methods (RMM) that will appear in European Pharmaceutical Review during 2012. As many of you know, I am always on the lookout for the next generation of rapid microbiological method (RMM) technologies and solutions. In this article, I have invited Noe Miyashita, a researcher from Hitachi Plant Technologies, to describe a novel ATP bioluminescence technology platform that she and her colleagues are currently working on. But in order to frame this discussion, it is appropriate to provide some background material on the fundamental basics of ATP bioluminescent methods.
ATP bioluminescence is the generation of light by a biological process, and is most recognised in the tails of the American firefly Photinus pyralis. First discovered in 1947 by William McElroy, he described the ATP bioluminescence reaction in which ATP (Adenosine Triphosphate) is enzymatically consumed to produce light. Specifically, in the presence of the substrate luciferin, the enzyme luciferase will use the energy from ATP to oxidise luciferin and release photons (light at a wavelength of 562 nanometres). The photons can then be detected and measured by a luminometer equipped with a photomultiplier tube. Figure 1 provides an illustration of this chemical reaction.


Because all living cells store energy in the form of ATP, this cellular component can be used as a measure of organism viability. If we capture the microorganisms of interest, release the ATP from within these cells and add the luciferin and luciferase reagents, we can measure the amount of bioluminescence generated. The sensitivity for ATP bio – luminescence (i.e., how many cells are required to detect a sufficient amount of photons or light) is usually between 100 – 1000 bacterial cells and a single yeast/mould cell. For this reason, when low numbers of bacterial cells are anticipated in a test sample, an enrichment step in media may be required to allow these cells to multiply and produce a sufficient level of ATP for subsequent detection.
If we can increase the level of sensitivity to where an enrichment step is not required in order to detect very low levels of micro – organisms, the time to result when using ATP bioluminescence systems may be significantly shortened. This is the basis for the work being conducted by my co-author.
Development of a new technology
A rapid and highly sensitive ATP system has been developed which utilises the same ATP methodology described above; however, a 100-fold increase in sensitivity is now attainable, primarily attributable to the complete automation of the sampling steps and enhancements to ATP measurement tech – nologies that result in lower background contamination and interference, and a significant improvement in signal detection.
This new platform is applicable for the detection of low levels of organisms, such as those that are found in airborne environments, but in order to realise the technology’s benefits, an appropriate sampling system and automation scheme is required.
Automation of the measurement process
The most common method for airborne environmental monitoring employs the use of impaction-based samplers and an agar-based medium. The agar would subsequently be incubated and enumeration of colony forming units (CFU) would follow. Currently available ATP RMMs can be utilised for this application; however, if low levels of airborne organisms are expected, an enrichment step of the collected microorganisms may be required in order to provide a sufficient level of ATP that can be detected. But it is also important to minimise or eliminate potential false positives that may result from adventitious contamination as a result of post-processing and/or transfer procedures to an enrichment medium. Even if a more sensitive RMM were developed that would not require an enrichment step, it is still necessary to devise a strategy to minimise contamination of the original sample during post-processing and measurement. This is even more important when the RMM requires the air sample (and any microorganisms that may be present) to be transferred into a liquid phase prior to ATP measurement. This is the case for the technology described in this article.
Because airborne bacteria that are captured on a solid surface (by an inertial collision method) are required to be transformed into a liquid sample for analysis in the new ATP RMM, automation of this process was the greatest challenge. In order to realise the automation of this step, a special gel phase transition carrier was developed which is automatically converted into a liquid sample within the instrument via heating. From this point, all processing steps are also performed automatically, including the introduction of the liquid matrix into the system, addition of reaction reagents and ATP measurement. Calibration and internal controls, such as sample blanks and ATP standards, are also utilised. Furthermore, because the system is set up to directly accept liquids, the RMM has the capability to extend its application reach much farther than just air monitoring.
Detection signal improvement
In addition to automating the post-sampling and measurement processes, the efficiency of the bioluminescence procedure and the light collection optical system was an important requisite of the new technology. This involved the development of a customised ATP reaction protocol and enhanced reagents, resulting in a significant improvement in bioluminescent signal detection, not only for vegetative cells, but also for spores. At the heart of the tech nology is a novel and highly sensitive optical element, which detects bioluminescence at a much lower level than conventional ATP systems.
Workflow
One cubic metre air samples are collected in a 10 minute period via an inertial collision sampler, which has been qualified to ISO 14698-1 standards. Microorganisms are trapped onto the gel-phase carrier, which is housed within a special sampling cartridge. If a liquid sample is to be evaluated, the sample is added directly to the sampling cartridge. Once inside the instrument, the gel material is melted by raising the temperature, and is subsequently removed via filtration. Then, a robotic system adds the ATP reaction reagents and introduces the resulting matrix into a measurement tube. A highly sensitive photomultiplier tube detects the resulting bioluminescence, and calculates the actual amount of ATP present in the sample, which is directly proportional to the amount of photons, or Relative Light Units (RLU), that are being quantified by the system.
By increasing the overall sensitivity of the system, no culturing or enrichment of the original sample is required. The system is capable of processing six samples and obtaining a quantitative measurement of ATP within two hours (from collection to measurement result).
Initial validation studies
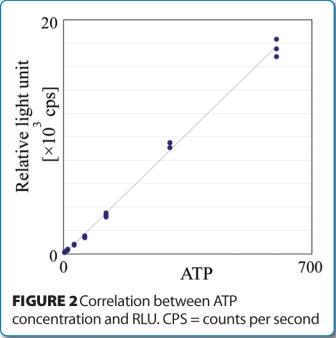
The lower limit of ATP detection was assessed by measurement evaluations of an ATP standard solution. Initial studies demonstrated an ATP calculated limit of detection (LOD) and limit of quantification (LOQ) equal to 1.3 attomole (amol) and 3.9 amol, respectively. Figure 2 shows the linear response between ATP concentration and RLU.


Because one bacterial cell has, on average, one attomole of ATP, these data demonstrate the RMM is capable of detecting a single viable cell, without the need for enrichment.
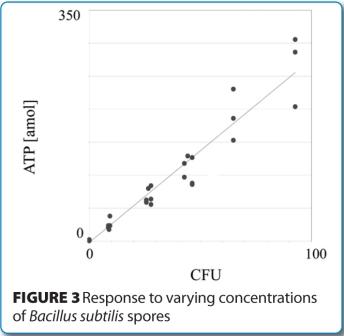
Similar measurement studies are currently under evaluation using low levels of a panel of standard strains of microorganisms, including bacterial spores. Initial experiments have demonstrated an excellent correlation and linearity between quantitative ATP measure – ments with the RMM and CFUs using conventional microbiological methods. Figure 3 provides an example of one such evaluation, where detected and quantitated ATP levels from low challenge concentrations of Bacillus subtilis spores are plotted against viable recovery rates (CFU) on conventional microbiological media.

Additional specificity testing is planned using sterilised bacteria to address the potential for detecting low levels of ATP and generating a false-positive result.
Summary
As RMMs continue to be developed, more and more technologies will offer greater sensitivity in detecting and quantifying microorganisms, without the need for microbial growth. ATP bioluminescence systems have certainly played an important role in moving the pharmaceutical industry toward adoption and implementation of 21st century microbiology methods, and the scientific advances that were discussed in this article provide encouragement that a variety of fresh and innovative options will continue to be introduced in the future.
About the authors
Dr. Michael J. Miller is an inter – nationally recognised microbiologist and subject matter expert in pharmaceutical microbiology and the design, validation and implementation of rapid microbiological methods. He is currently the President of Microbiology Consultants, LLC (http://microbiologyconsultants.com). Over the course of 25 years, he has held numerous R&D, manufacturing, quality, and consulting and business development leadership roles at Johnson & Johnson, Eli Lilly and Company, Bausch & Lomb, and Pharmaceutical Systems, Inc. In his current role, Dr. Miller consults with multinational companies in providing technical, quality and regulatory solutions in support of RMMs, sterile and non-sterile pharmaceutical manufacturing, contamination control, isolator technology, validation and microbiological PAT. He also provides comprehensive training for his clients in the areas of rapid method validation and implementation.
Dr. Miller has authored more than 100 technical publications and presentations in the areas of rapid microbiological methods, PAT, ophthalmics, disinfection and sterilisation, is the editor of PDA’s Encyclopedia of Rapid Microbiological Methods, and is the owner of http://rapidmicromethods.com, a website dedicated to the advancement of rapid methods. He currently serves on the editorial board for European Pharmaceutical Review, is co-chairing the revision of PDA Technical Report #33: Evaluation, Validation and Implementation of New Microbiological Testing Methods, and routinely provides RMM training programs for the industry and professional organisations worldwide.
Dr. Miller holds a PhD in Microbiology and Biochemistry from Georgia State University (GSU), a BA in Anthropology and Sociology from Hobart College, and is currently an adjunct professor at GSU. He was appointed the John Henry Hobart Fellow in Residence for Ethics and Social Justice, awarded PDA’s Distinguished Service Award and was named Microbiologist of the Year by the Institute of Validation Technology (IVT).
Noe Miyashita received her MS in Life Science (Molecular Biology) from Tokyo University. In 2005, she joined Hitachi Plant Technologies, Ltd., which is the engineering and construction company in the Hitachi group. She is the researcher responsible for technologies for bio clean rooms, such as air conditioning and microbiological control facilities. Her most recent interests lie in the development of RMM technologies where she has found collaborators both within Hitachi, Ltd. as well as with universities and national laboratories in Japan.