Enthalpic efficiency and the role of thermodynamic data in drug development: possibility or a pipeline dream!
Posted: 23 November 2007 | Professor John E. Ladbury, Department of Biochemistry and Molecular Biology, University College London | No comments yet
The determination of accurate thermodynamic data for the interactions of biomolecules has been enhanced over the last decade by the use of isothermal titration calorimetric (ITC) instrumentation. These instruments are now standard kits in many biophysical/structural biochemistry laboratories of pharmaceutical companies. Despite this, there is little evidence for the input of thermodynamic data into the drug development process.
The determination of accurate thermodynamic data for the interactions of biomolecules has been enhanced over the last decade by the use of isothermal titration calorimetric (ITC) instrumentation. These instruments are now standard kits in many biophysical/structural biochemistry laboratories of pharmaceutical companies. Despite this, there is little evidence for the input of thermodynamic data into the drug development process.
The determination of accurate thermodynamic data for the interactions of biomolecules has been enhanced over the last decade by the use of isothermal titration calorimetric (ITC) instrumentation. These instruments are now standard kits in many biophysical/structural biochemistry laboratories of pharmaceutical companies. Despite this, there is little evidence for the input of thermodynamic data into the drug development process.
The panacea of being able to correlate thermodynamic parameters with structural perturbation on going from the free state to the target-drug complex is proving difficult to obtain, and as such the value of calorimetric data has been largely disregarded. As a result it is important to ask whether thermodynamic data can provide added value to the drug development process, and if so at what stage can this input be made? In this article the calorimetric method is introduced and evaluated as a platform technology for drug development. The potential to input thermodynamic data in the drug development pipeline is assessed. The conclusion is that these data add significantly to the decision making process at the lead selection and optimisation stage.
Isothermal titration calorimetry (ITC) has become a standard, commercially available laboratory instrument which adds to the arsenal of other methods from which thermodynamic parameterisation of biomolecular interactions can be determined. The method has been described in detail elsewhere1-4 (see Figure 1). Highly accurate determination of the thermodynamic parameters of a biomolecular interaction can be obtained with no requirement for chemical modification or limit on size of interacting components. The direct measurement of the change in heat (enthalpy, ΔHobs) provides a probe of the extent of complex formation occurring throughout a titration of two components of known concentrations. Since calorimetric methods directly measure the observed ΔHobs of an interaction at a given temperature, they avoid the necessity of calculation of this term via the van’t Hoff relationship which is required when adopting other methods (for example, spectroscopic or surface plasmon resonance techniques) and is inherently less accurate. In the ITC experiment the heat of interaction measured includes contributions from all the equilibria that occur on the interacting components going from the free to bound state. Thus, the thermodynamic terms are considered as ‘observed’ to signify that they are derived from experiment and the subscript ‘obs’ is inserted.
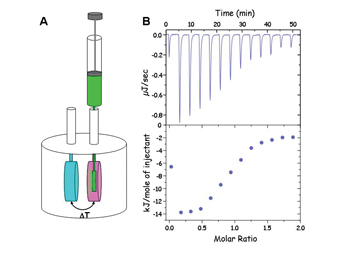

Figure 1: A. Schematic of isothermal titration calorimeter. Two cells are held in an adiabatic jacket which is set at a temperature below the experimental temperature. The blue cell is a reference cell. The pink cell is the reaction cell. Injections of one component are made via the syringe into the other component. Heat change is measured with respect to the reference cell. The change in heat is compensated for by increasing (endothermic) or decreasing (exothermic) the power into the reaction cell. These power changes are recorded as shown in B. B. Top panel shows raw data for 13 injections showing power versus time. Lower panel shows integrated raw data. As more injections are made the heat per injection (the integrated power with respect to time) becomes less as the binding sites become saturated. The first injection is lower volume to remove effects of dissolution. For experimental details see ref. 4
Over the course of a titration the amount of free/bound interactants can be determined and thus the observed equilibrium binding constant, KB,obs (= 1/KD) can be obtained. Having established the ΔHobs and KB,obs parameters at a given experimental temperature (T) the observed change in free energy, ΔGobs can be determined using equation 1:
ΔGobs = -RT•lnKB,obs (1)
Furthermore, knowing the ΔGobs and the ΔHobs the change in entropy, ΔSobs, can be calculated, from equation 2:
ΔSobs = (ΔHobs – ΔGobs)/T (2)
The enthalpy is measured at a range of temperatures then the change in constant pressure heat capacity (ΔCp,obs) can be determined based on the following equation:
ΔCp,obs = (ΔHT1,obs – ΔHT2,obs)/(T2 – T1) (3)
(where T1 and T2 are two different experimental temperatures).
The ITC method thus enables the rapid determination of a complete thermodynamic profile of an interaction in one experiment. With the ready availability of this information, one might have expected that these data would have made an impact not only in understanding biological systems, but in the area of structure-based drug development. However, this has yet to be realised.
One cornerstone of biophysical scientific endeavour is to be able to correlate the structural perturbation which accompanies biomolecular interactions (for example, the making and breaking of non-covalent bonds between the interacting macromolecules, as well as with the solvent, and the accompanying structural conformational rearrangements) with the thermodynamic quantity associated with these changes. From this correlation the panacea of being able to predict the thermodynamic character of an interaction, especially the free energy (or affinity), from some knowledge of the structure of the binding site and the potential ligand was very attractive to the drugs industry. For example, this would enable calculation of thermodynamic properties of an interaction of a range of small molecules with a target molecule if the high resolution structure of these was known.
Unfortunately, this panacea has remained elusive. This is largely because although structural determination provides atomic detail of the interacting molecules, ITC measurement reports on changes which include the formation of non-covalent interactions in the complex, but also changes in solvation, which are not described by the high resolution structure.
That is to say that if the formation of a biomolecular complex involved only the formation of a defined number of hydrogen bonds between atoms observable in the structure, then the thermodynamic parameters measured for the interaction could be attributed to this and correlations easily made. However, when a ligand binds a number of events are possible; firstly, non-covalent bonds form between the ligand and the biomolecule, secondly, non-covalent bonds break between the solvent and the biomolecular binding site which becomes buried by the interacting ligand and finally, similar types of bonds break between the solvent and the ligand. In addition, there are other effects such as; conformational change, protonation and ion binding which could also contribute to the change in thermodynamic parameters for the interaction. As a result, there are too many undefined events occurring in a single binding event which cannot be revealed by the high resolution structure, but which are included in the measured thermodynamic parameters.
The most refined attempts to correlate structure and thermodynamic parameters have been based on the perceived relationship between the burial of surface area and the change in constant pressure heat capacity, ΔCp. The original observations were made on protein folding/unfolding equilibria and heats of desolvation of small hydrocarbon compounds where it was presumed that the ΔCp effect was due to the release of organised water molecules on hydrophobic surface5-7. This correlation with hydrophobic surface has progressively been fine-tuned to include hydrophilic surface and increasingly detailed definitions of surface. Although there is some value in these structure/thermodynamic correlations, there are many examples of lack of correlation and indeed questions over the whole tenet of the approach8,9. This lack of ability to directly correlate structure and thermodynamic parameters and hence make useful predictions about the binding characteristics of lead compounds has in many cases led to pharmaceutical scientists ignoring the potential value of these parameters.
So where does the added value in thermodynamic input to drug development arise? The use of ITC is not really suggested for the initial library screening. Even with the latest technology in ITC instrumentation (see for example http://www.microcal.com/ and http://www.calscorp.com/) the ability to screen only tens of samples per day (even on an automated stage and by single injection methods) means that high throughput will never be attainable. Spectroscopic methods will always have higher sensitivity. In any case, the requirement for a full thermodynamic characterisation of an interaction at the screening stage is not clear. Thus the strength of calorimetric data is found further down the drug development pipeline.
The major value in adopting ITC is in the ability to utilise the high quality thermodynamic data. Although ITC provides the ability to quantify the affinity of a given compound through the ΔG term, since most hits from screening have affinities within one or two of orders of magnitude of one another (which corresponds to only ~6 kJ.mol-1 of ΔG), this information may have limited value in the decision making progress. Thus we suggest that added value from ITC data in the drug development process can be derived from measurement of the ΔH term.
ΔH represents a direct readout of the heat associated with the making and/or breaking of non-covalent bonds on complex formation. Thus in its simplest form, if the binding of a ligand is accompanied by a favourable ΔH, then more or stronger bonds exist in the bound state than in the free state. This is important because in the rational development of ligands towards drugs it is extremely difficult to engineer novel non-covalent bonds. For example, there are very few reported cases where a compound has been modified successfully to create a novel hydrogen bond which contributes in a predictable way to the affinity with the protein target. The formation of hydrogen bonds are dictated by atomic distances and orientation of donor or acceptor atoms within the binding site. To modify a small molecule to satisfy these exquisitely sensitive atomic requirements is difficult. Thus for the most part, formation of novel hydrogen bonds in structure-based compound development occurs serendipitously. It is generally considered easier to increase the entropic component to the ΔG. This can generally be done by increasing the area of hydrophobic surface on the compound which can be buried on forming the complex with the target. Removal of hydrophobic surface area increases the entropy term in line with release of interacting water molecules.
Clearly, taken in isolation the measurement of ΔH is not of much use since the net change in bonds covers not only those formed/broken in making the complex, but also those derived from solvent. The value of the ΔH term is found when comparing similar molecules which bind to the same target binding site (for example, measuring ΔΔHobs). This is because if the compounds are similar, the effects of removal of solvent from the burial of surface area will largely cancel out. Thus if a series of ‘hits’ from a screen have been identified, one criteria for a judgement on which ones are suitable for further optimisation would be based on those with the comparatively most favourable enthalpic contribution to binding10,11. This adoption of the ΔH term as part of the decision making process in selection of leads can be considered similar to the use of ‘ligand efficiency’ which is used by medicinal chemists to rate a molecule. Ligand efficiency is based on the number of bonding groups/molecular weight of the compound. Thus, ‘enthalpic efficiency, EE,’ (for example the DH/molecular weight of the compound) might be a better yardstick by which medicinal chemists can compare their worth since it provides a quantification of the ligand’s net bond forming capability. Clearly it would be foolhardy to adopt these data at the expense of other input from tried and tested criteria such as those reported by Lipinski12, however, the EE should add to the tools for the decision making process.
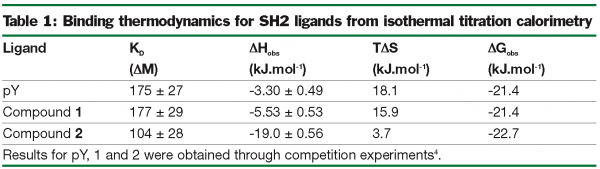

The value of EE is demonstrated in the compounds derived from the fragment library screen13 shown in Table 1. This work was based on the identification of low molecular weight compounds from a fragment library which would bind to the phopshotyrosine, pY, binding pocket of the SH2 domain from the Src protein14. These could provide precursor molecules to enable development of novel inhibitors for the interaction of Src with physiological cognate ligands. The initial in silico screen of ~13000 compounds was followed by a 19F NMR screen of selected compounds. The Table shows the ITC-derived data for the binding of two of these fragments. The physiological ligand (pY) and the fragments (compounds 1 and 2) have similar affinities as reflected in the ΔGobs. Based on the ΔHobs values a decision was reached as to which compound is suitable for further molecular modulation by the medicinal chemists. Compound 2 clearly has a significantly more favourable ΔH, which suggests that it is making a better complement of non-covalent bonds in the pY binding site than compound 2 and the pY moiety itself. The improved EE for compound 2 is likely to be derived from the additional hydrogen bond(s) shown in Figure 2.
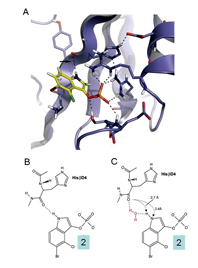

Figure 2: A Ribbon representation of the pY binding site (purple). Compound 2 (stick representation) is modelled into this site. Hydrogen bonds are shown as dotted lines. B and C show schematic representations of the ligand modelled into the pY binding site (shown above) highlighting potential novel hydrogen bonds either directly to A an histidine residue or B by mediation by a water molecule.
One potential further attraction of the use of the ΔHobs in aiding compound selection is that it is possible to derive the data from a single (or ideally small number) of injections in the ITC experiment. The DH of the interaction can be determined from the binding of a known concentration of ligand to the target molecule. Thus if the concentration regime is set up such that in the first injection all of the ligand binds (Figure 1B), then the heat from this injection corresponds to the change in enthalpy per mole of ligand. This potentially reduces the amount of material required, particularly in the case of higher affinity measurements.
The input of now readily accessible thermodynamic data to the drug development process has not been forthcoming. This is largely a result of the inability to reliably correlate structural perturbation occurring on complex formation with the thermodynamic parameters measured. However, the comparison of ΔHobs for formation of complexes of similar compounds to the same binding site provides added value to the decision making process in selection of ligands for further development. Since the pipeline of events which involves identifying and developing a lead compound to a marketable drug is extremely expensive, both temporally and financially, all data which can enhance the decision making process has to be valued; thus thermodynamic data is ignored at peril.
References
- T. Wiseman, S. Williston, J. F. Brandts, L. N. Lin, Anal. Biochem. 179, 131, 1989.
- J. E. Ladbury, Structure 3 635, 1995.
- J. E. Ladbury, B. Z. Chowdhry, Chem. & Biol. 3, 791, 1996
- J. E. Ladbury, & M. L. Doyle. Biocalorimetry II: Applications of Calorimetry in the Study of Biological Systems. John Wiley & Sons Ltd. Chichester, Sussex, UK. ISBN 0-470-84968-1. 2004
- J. R. Livingstone, R. S. Spolar, M. T. Record, Biochemistry, 30, 4237-4244, 1991
- R. Spolar, M. T. Record, Science. 263, 777-783 1994
- J. Gomez, E. Freire, in J. E. Ladbury and P. R. Connelly (Eds.) Structure-based Drug Design: Thermodynamics, Modeling and Strategy. Springer (R. G. Landes) Berlin, Germany. p. 111. 1997
- Morton, C. J., & Ladbury, J. E. Protein Science 5, 2115-2118. 1996.
- A. Cooper, C. M. Johnson, J. H. Lakey, M. Nollmann Biophys. Chem.93, 215-230. 2001.
- I. Luque, E. Freire. Proteins: Struct., Funct. Genet. 49, 181-190, 2002.
- A. J. Ruben, Y. Kiso, E. Freire E. Chem Biol. Drug. Des. 67, 2-4, 2006.
- C. A. Lipinski F. Lombardo, B. W. Dominy P. J. Feeney Adv. Drug. Deliver. 46, 3-26. 2001.
- J. D. Taylor, P. J. Gilbert, M. A. Williams, W. R. Pitt, J. E. Ladbury, Proteins: Struct. Funct. Bioinf. 67, 981-990. 2007.
- D. A. Henriques, J. E. Ladbury. Arch. Bioch. Biophys. 390, 158-168. 2001.
Professor John E. Ladbury
Department of Biochemistry and Molecular Biology, University College London
Professor Ladbury is currently Professor of Molecular Biophysics and a Wellcome Trust Senior Research Fellow in the Department of Biochemistry and Molecular Biology at University College London (UCL). A graduate in chemistry and physics from the University of London, he went on to receive his PhD in inorganic polymer chemistry as a Ministry of Defence Research Scholar from the University of Greenwich in 1990. Professor Ladbury held postdoctoral appointments at Yale University, Harvard University Medical School and New York University Medical Center before taking up a Wellcome Trust Career Development Research Fellowship at the University of Oxford in 1995. Professor Ladbury received a Wellcome Trust Senior Fellowship after moving to UCL. His laboratory at UCL adopts a multi-disciplinary approach to understanding thermodynamic/structural correlation in interactions of proteins. His group are currently involved in drug development projects on inhibitors of intracellular signalling pathways and novel antibacterial targets.




