ESC Congress 2013: New data provide evidence for the positive safety and efficacy profile of Pradaxa® in various atrial fibrillation patient populations
Posted: 26 August 2013 | | No comments yet
Boehringer Ingelheim announced the upcoming presentation of new data on the novel oral anticoagulant (NOAC) Pradaxa® (dabigatran etexilate) during the ESC Congress 2013…


Boehringer Ingelheim today announced the upcoming presentation of new data on the novel oral anticoagulant (NOAC) Pradaxa® (dabigatran etexilate) during the ESC Congress 2013 organised by the European Society of Cardiology (August 31 – September 4, Amsterdam, The Netherlands). The new data will add to the already available extensive body of knowledge on Pradaxa®’s positive safety and efficacy profile.
Boehringer Ingelheim remains committed to advancing the cardiology field and supporting physicians in their search for optimal stroke protection. Data being presented at the ESC Congress 2013 will complement the ongoing clinical trial programme of Pradaxa®, including results from the pivotal RE-LY® trial and the only long-term NOAC study, RELY-ABLE®, as well as new preclinical research on the company’s investigational antibody fragment antidote for dabigatran.
Professor Klaus Dugi, Corporate Senior Vice President Medicine, Boehringer Ingelheim
“The breadth of data being presented for Pradaxa® at this year’s ESC Congress 2013 demonstrates Boehringer Ingelheim’s continued dedication to improving the lives of patients with cardiovascular disorders. Our extensive and ongoing investment into clinical research will provide additional evidence on the positive efficacy and safety profile of Pradaxa® as well as important insights on its use in a wide range of patients and clinical settings”, said Professor Klaus Dugi, Corporate Senior Vice President Medicine, Boehringer Ingelheim.
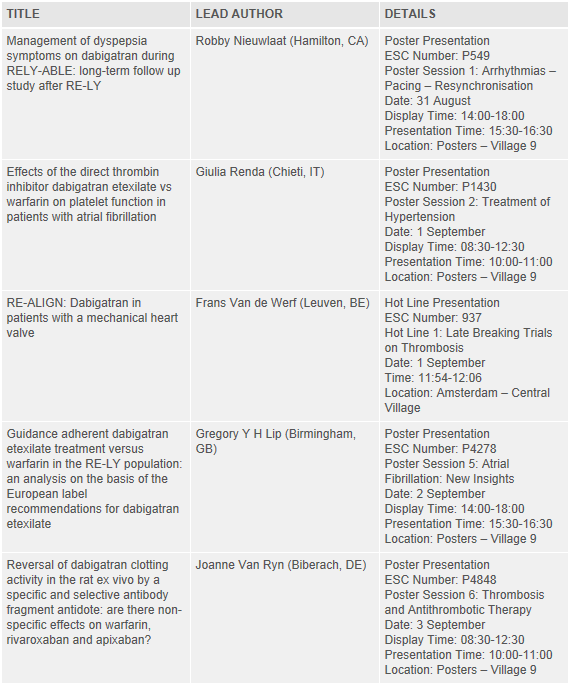
Details of the key Pradaxa® abstracts being presented at the ESC Congress 2013 are listed below. Further information and the full list of abstracts can be found within the Scientific Programme, available at: http://spo.escardio.org/default.aspx?eevtid=60
Pradaxa® is widely approved for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation and for primary prevention of VTE following total hip replacement or total knee replacement surgery.1 The extensive in-market experience of over 2 million patient-years in all licensed indications puts Pradaxa® first among the novel oral anticoagulants.2 In addition, Boehringer Ingelheim recently started submitting applications to regulatory authorities for the use of Pradaxa® in the acute treatment and prevention of recurrent deep vein thrombosis and pulmonary embolism.

About Pradaxa® (dabigatran etexilate)
Pradaxa® is approved in over 100 countries worldwide.2 It is licensed for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation and for the primary prevention of venous thromboembolism in patients undergoing total hip replacement or total knee replacement surgery.1
Pradaxa®, a direct thrombin inhibitor (DTI),3 was the first of a new generation of direct oral anticoagulants targeting a high unmet medical need in the prevention and treatment of acute and chronic thromboembolic diseases.
Potent antithrombotic effects are achieved with direct thrombin inhibitors by specifically blocking the activity of thrombin (both free and clot-bound), the central enzyme in the process responsible for clot (thrombus) formation. In contrast to vitamin-K antagonists, which variably act via different coagulation factors, dabigatran etexilate provides effective, predictable and consistent anticoagulation with a low potential for drug-drug interactions and no drug-food interactions, without the need for routine coagulation monitoring or dose adjustment.
References
1Pradaxa European Summary of Product Characteristics, 2013
2Boehringer Ingelheim data on file.
3Di Nisio M, et al. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028-40.



