Calorimetry for amorphous content quantification
Posted: 23 May 2007 | | No comments yet
In the previous article (European Pharmaceutical Review, Issue 2, 2007) an introduction to calorimetry was given and its application to polymorph characterisation, discussed. Another area of application of growing importance is quantification of (usually small) amorphous contents. A requirement to demonstrate the presence or absence of amorphous material is becoming more important in regulatory documentation and calorimetric techniques are emerging as major tools in this arena. This article focuses on the use of various calorimetric techniques for quantifying amorphous content.
In the previous article (European Pharmaceutical Review, Issue 2, 2007) an introduction to calorimetry was given and its application to polymorph characterisation, discussed. Another area of application of growing importance is quantification of (usually small) amorphous contents. A requirement to demonstrate the presence or absence of amorphous material is becoming more important in regulatory documentation and calorimetric techniques are emerging as major tools in this arena. This article focuses on the use of various calorimetric techniques for quantifying amorphous content.
In the previous article (European Pharmaceutical Review, Issue 2, 2007) an introduction to calorimetry was given and its application to polymorph characterisation, discussed. Another area of application of growing importance is quantification of (usually small) amorphous contents. A requirement to demonstrate the presence or absence of amorphous material is becoming more important in regulatory documentation and calorimetric techniques are emerging as major tools in this arena. This article focuses on the use of various calorimetric techniques for quantifying amorphous content.
Amorphous content determination
Understanding and quantifying any amorphous material in a sample is of increasing importance because of the significant effects it can exert. For instance, deliberate inclusion of the amorphous form of a drug can be a formulation strategy used intentionally to increase the dissolution rate of a poorly soluble active.1 Conversely, mechanical processing of solid pharmaceuticals may result in the accidental formation of amorphous regions in what was previously an entirely crystalline material.2 Despite the amorphous material typically occupying only a few percent of the bulk mass, the fact that it is sited on the surface of the particles affords it disproportionate control over the surface interactions of what is a predominantly crystalline powder.3-4
In either situation, the amorphous content may decrease the observed physical and/or chemical stability of the formulation. The Food and Drug Administration’s (FDA) Drug Substance Guideline states that “appropriate” analytical procedures should be used to detect polymorphic, hydrated or amorphous forms of the drug substance.5 Consequently, the detection, quantification, characterisation and management of amorphous contents, particularly small amorphous contents, play a central role in ensuring process control during development and manufacturing stages. Many forms of calorimetry allow quantification of amorphous contents and each method has specific benefits and drawbacks.
The use of isothermal calorimetry (IC) for amorphous content determination in excipients (for instance,6-11) and drugs (for example,9,12-16) is becoming more widespread. The methodology is simple; the heat is measured as the sample is plasticised (usually with water but any appropriate vapour will suffice) and then crystallises. The methodology is thus predicated on the assumption that the measured heat of crystallisation is proportional to the amorphous content, but there are a number of factors that mean this may not necessarily be true.
The first concerns the method of introducing vapour; in many cases, the sample under investigation is placed into a glass ampoule (hygrostat) and exposed to a specific RH (Relative Humidity) by means of a hygrostat containing a saturated salt solution (saturated salt solutions maintain a specific RH at a particular temperature). The ampoules are sealed and placed in the calorimeter. A number of events then proceed; water evaporates from the hygrostat (assuming the RH being maintained is above ambient) and wetting of the internal surfaces of the ampoule and sample occurs, the amorphous regions absorb water and plasticise; the amorphous material crystallises, which results in the expulsion of water from the crystal lattice, and water condenses back into the reservoir in order to maintain equilibrium. Since each of these processes will allow heat change to rise, it is apparent that the calorimetric signal will be complex and comprise a number of components. Note also that some of these events are exothermic (crystallisation, wetting and condensation) while others are endothermic (evaporation) and that the calorimeter records only net change in heat. Note also that hydration is initiated externally from the calorimeter (and, hence, the initial wetting, and possibly some crystallisation, data are lost), which is an issue for samples that crystallise over short (1-2 h) time spans.
Thus, a typical crystallisation response contains a number of exothermic and endothermic processes, giving a composite trace that consists of several peaks or shoulders on peaks. This makes definition of the starting and ending points of the crystallisation process itself difficult and open to subjective variation. Dilworth et al11 discussed the effect of selecting different integration strategies and the consequences for the calculated amorphous content; of the 12 integration strategies presented, only 2 were deemed to be suitable for amorphous content determination, integration of the entire response or integration of solely the main crystallisation peak. Analysis of the data showed that the former strategy gave the most repeatable data.
An alternative method of controlling the RH in the sample cell is RH perfusion. Here, two gas lines are routed into the ampoule; one passes through a number of humidifying chambers and is hence saturated with vapour by the time it reaches the ampoule and the other passes through a desiccant and is hence dry. Proportional mixing of the two gas streams (usually by mass-flow controllers, although pulsed mixing may also be used) produces any desired RH in the sample chamber. Forms of isothermal RH perfusion calorimetry have been used to study the water adsorption properties of amorphous ceftidoren pivoxil,2 study powder surface energetics17 and assess the physical stability of amorphous cimetidine.18
This experimental methodology opens an alternative strategy for determining small amorphous contents. The sample can be held under a pre-set RH programme (for instance, 0-90-0% RH); this results in calorimetric data that represent the wetting of the partially amorphous sample and the drying of the (now) entirely crystalline sample. The crystallisation response of the amorphous fraction is thus given by subtracting the drying response from the wetting response. One clear advantage of this approach, over the hygrostat method discussed above, is that the wetting responses of the ampoule and the (majority of the) sample are automatically removed. Note here that the sample that wets, partially amorphous, is different from the sample that dries, entirely crystalline, so this approach is subject to a small experimental error, although this will diminish with lower amorphous contents. Ramos19 provides an analysis of this type of measurement.
Changes in the experimental conditions can also have an impact upon the observed response in the microcalorimeter, because the balance of events occurring is altered, and must be taken into account. For instance, experiments on lactose conducted at 60°C resulted in a larger enthalpy of crystallisation, because less plasticising water needed to be absorbed and subsequently expelled.20 Selection of RH, temperature and sample mass can also impact the measurement. The RH generated by the saturated salt solution has to be sufficient to lower the Tg to the experimental temperature. In addition, at each measurement temperature, a balance between sample mass and RH must also be achieved so that the lag time for crystallisation is long enough to allow thermal equilibration of the ampoules before crystallisation starts to take place. In most cases an exothermic water sorption event is observed prior to crystallisation. In order to facilitate data analysis, the chosen RH/sample mass combination should also allow a lag phase between the first exothermic event and the crystallisation signal. It is important that any report of an amorphous content determination specifies exactly the experimental conditions employed.
The use of solution calorimetry (an example system is shown in figure 3) for amorphous content determination is predicated on the fact that the enthalpy of solution (ΔsolH) of a particular solute may be affected by minor changes of its physico-chemical properties. In particular, a marked difference is often noted for crystalline and amorphous forms of the same material, which derives from the fact that an amorphous material has no lattice energy while a crystalline material does. Indeed, it is often the case that dissolution of a crystalline form is endothermic while dissolution of an amorphous form is exothermic. In a similar manner to the case for polymorphism discussed in the previous article, the measured heat of solution thus results from the sum of the enthalpies and weight fractions for the crystalline and amorphous states present in the solute, according to the following relationship:21
Where XI and XII are the fractions of the amorphous and crystalline material and ΔsolHI and ΔsolHII are the enthalpies of solution of the amorphous and crystalline material respectively. This being so, a plot of ΔsolH versus percent amorphous content should be linear.22-26 As ever, many published investigations have used lactose as a test substance; Hogan and Buckton22 report that the use of SC(Solution calorimetry) for the quantification of amorphous content in lactose between 0 and 10% is good to ± 0.5%, while Harjunen et al23 recently determined limits of detection and quantification of amorphous lactose of 1.8% and 6.0% respectively.
Although it operates on a simple principle, the use of solution calorimetry is complicated by several factors and it is the case that while it is a valuable tool for assessing small amorphous contents, its use must be tempered by careful experimental design and sample preparation.
The first issue is that the sensitivity of the technique depends entirely upon the difference in the ΔsolH values between the amorphous and crystalline forms. It is thus only possible to state a sensitivity of measurement for a specific sample and the approach is more suited to some samples than others. Furthermore, it was noted earlier that the amorphous form often has an exothermic ΔsolH while the crystalline form has an endothermic value. For materials where this is so, it can be seen that the calibration curve will, at some composition, cross zero on the y-ordinate. The calorimeter will thus measure no net signal at this composition and its sensitivity either side of this value will be reduced.
In order to calculate the value of ΔsolH in normalised units (Jmol-1 or Jg-1) the solute must be freely soluble in the volume of solvent used (although surprisingly a recent study showed that it is possible to use SC for quantification of amorphous content even when the sample is only slightly soluble in the solvent).23, 26
Another limitation of the method is that the samples need to be completely dry. The higher the amount of water in the sample, the lower the wetting response (exothermic); as a consequence, the enthalpy of solution will be less exothermic.21-22 If protection of samples from atmospheric moisture is essential, a long drying period might lead to aging of the amorphous material. In this event the enthalpy of solution becomes more endothermic upon aging.21 Consequently, both amount of water present and aging of the amorphous material will almost certainly impact on the quantification of amorphous content. (Typical data that would be obtained from a solution calorimetry experiment is shown in Figure 4).
Using DSC(temperature-modulated differential scanning calorimetry), the traditional approach for the quantification of amorphous content is based on determination of the enthalpy of fusion. When the enthalpy of fusion of an entirely crystalline sample is known, the amorphous content is determined from the difference between a 100% crystalline sample and the test sample. This methodology has been applied for the quantification of amorphous content of polymers27 and lactose.28 A drawback can be the determination of the area of the peak of fusion, because it can be difficult to fix the integration limits; in the case of polymers because melting peaks can start around 50°C below the maximum temperature of the peak27 and in the case of anomeric mixtures because melting peaks may be superimposed.11 When determining the crystallinity of α-lactose monohydrate by conventional DSC, Gombás et al verified a variation of the areas of the melting peaks of lactose (β- and α-lactose melting peaks) but could not establish a relation between crystallinity and these peaks.28
Another approach for the quantification of amorphous content using DSC is to determine the change of heat capacity (ΔCp) at the glass transition temperature (Tg). When ΔCp at Tg is used to quantify amorphous material, it has to be ensured that the transition for wholly amorphous material is reproducible, so that it can be used as a reference value. The ΔCp is affected by a number of factors, such as structural relaxation of the amorphous material29 and relaxation time.30 Furthermore, on a DSC trace, the enthalpy recovery exotherm is normally superimposed on Tg, which complicates the accurate determination of the DCp value. The exact value of Tg is largely affected by the water content of the sample and experimental conditions.31-32
Many authors have tried to establish a methodology for quantification of amorphous content by DSC through the use of changes in heat capacity (for instance;29-30,33-34). Both TMDSC (temperature-modulated differential scanning calorimetry) and FS-DSC (fast-scan differential scanning calorimetry) offer potential for these measurements. Analysis of TMDSC data allows the separation of ‘reversible’ events from ‘non-reversible’ events; from the point of view of ΔCp measurements, this is particularly fortuitous because it results in the glass transition step change being separated from the enthalpy relaxation, making a true assessment of the height of the step-change (and hence measurement of ΔCpp) much easier. Saklatvala et al35 showed that quantification to 5% w/w amorphous content was easily achievable with TMDSC. Guinot and Leveiller34 employed the same method to determine the amorphous content in samples of a micronised drug substance. The authors showed it was possible to construct a calibration curve of ΔCp against known amorphous content. Limits of detection and quantification of 0.9 and 3.0% w/w respectively were obtained.
FS-DSC has been reported to increase significantly the sensitivity to amorphous content.29-30, 33, 36 The suitability of the method for quantification of amorphous lactose33 and amorphous maltitol30 down to ca. 1% w/w and for amorphous sucrose29 down to ca. 0.2% w/w has been demonstrated.
Again, control of sample composition is paramount. Yoshioka et al37 showed that two different batches of amorphous indomethacin exhibited similar glass transition temperatures. However, one of the batches exhibited a much greater annealing endotherm than the other. Although a difference in heat capacity at Tg was not mentioned, one might expect it to differ between batches (to our knowledge, there are no reports in the literature of any evidence that the change in heat capacity at Tg is affected by the polymorphic/ anomeric composition of the samples).
A final approach for the quantification of amorphous material by DSC is based on the determination of the enthalpy of crystallisation. This is predicated on the fact that when a fully or partially amorphous sample is subjected to a heating programme, at a certain temperature between its Tg and melting it will crystallise and a crystallisation exotherm will be seen; the higher the amorphous content, the larger the enthalpy of crystallisation. There are a number of reports of this methodology in the literature; Saleki-Gerhardt et al38 were able to quantify amorphous sucrose with an overall sensitivity of ± 5% down to ca. 10% w/w amorphous content. In that study, a heating rate of 10°Cmin-1 was used. In a recent study, the determination of the enthalpy of crystallisation by DSC was one of the reference methods for the quantification of amorphous content.39 Lactose was used as the model substance to create a calibration curve based on predetermined mixtures of highly crystalline and amorphous substance. The obtained calibration curve showed a clear negative deviation from linearity. The authors attributed it to an incomplete crystallisation of the amorphous portion or to the fact that non-hermetically sealed pans were used. Using the same methodology, Gombás et al28 reported a linear relation between the measured enthalpy and crystallisation and the crystallinity of lactose samples. However, if their data are fitted to a non-linear model, the correlation is better than when fitted to a linear model.
Summary
Calorimetric techniques are ideally suited to quantification of amorphous content because they offer a bulk measurement (i.e. the measured response is that of the entire sample). Quantification is possible via a number of different calorimetric derivatives, which means it is usually possible to find at least one methodology that gives an acceptable level of quantification, often to 0.5% w/w. A further benefit of calorimetry, not discussed here, is that measurements on relaxation of amorphous materials are also possible. Thus it becomes possible not only to quantify the amount of any amorphous material present within a sample but also to characterise it (data which demonstrate shelf-life, for instance). The growing need to understand and control, and the increasing desire to formulate with, the amorphous form means that interest in this area can only increase; calorimetric techniques can play a central role in this area.
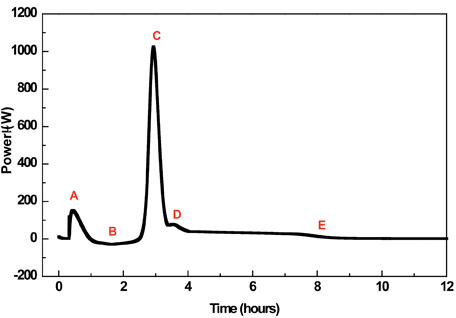

Figure 1: A typical power-time response for a sample of amorphous lactose held at 25°C under an RH of 75%. The data comprise several events (discussed in the text) and the best strategy integration is not always clear. Reproduced from (11) with permission from Elsevier Science.
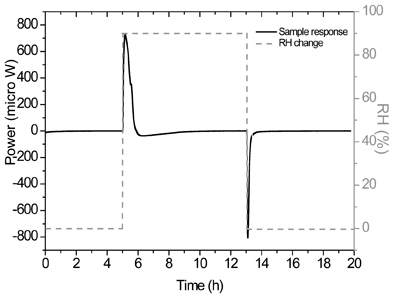

Figure 2: The wetting and drying calorimetric response of a 5% w/wamorphous sample of a-lactose using RH perfusion calorimetry. Reproduced from (19) with permission from Elsevier Science.


Figure 3: A typical solution calorimeter. Image courtesy of Thermometric Ltd.
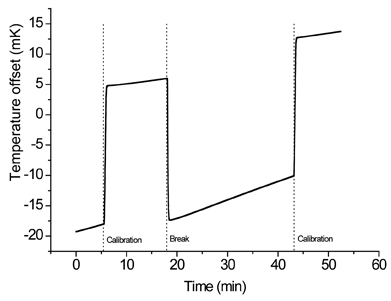

Figure 4: Raw solution calorimeter data showing the dissolution of a 5% w/w amorphous sample of a-lactose into water and the two electrical calibrations
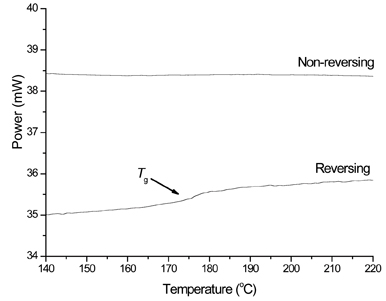

Figure 5: The separation of raw TMDSC data into reversing and nonreversing components.
References
- Ohara T, Kitamura S, Kitagawa T, Katsuhide T. Dissolution of poorly water-soluble drug from extended release solid dispersion system with ethylcellulose and hydroxypropylmethylcellulose. Int J Pharm, 302:95-102, 2005.
- Ohta, M, Buckton G. Determination of the changes in surface energetics of cefditoren pivoxil as a consequence of processing induced disorder and equilibration to different relative humidities. Int J Pharm, 269:81-88, 2004.
- Newell HE, Buckton G, Butler DA, Thielmann F, Williams DR. The use of inverse phase gas chromatography to measure the surface energy of crystalline, amorphous and milled lactose. Pharm Res, 18:662-666, 2001.
- Newell HE, Buckton G, Butler DA, Thielmann F, Williams DR. The use of inverse phase gas chromatography to study the change of surface energy of amorphous lactose as a funstion of relative humidity and the process of collapse and crystallisation. Int J Pharm, 217:45-56, 2001.
- Byrn S, Pfeiffer R, Hoiberg C, Poochikian G. Pharmaceutical solids: a strategic approach to regulatory considerations. Pharm Res, 12:945-954, 1995.
- Sebhatu T, Angberg M, Ahlneck C. Assessment of the degree of disorder in crystalline solids by isothermal microcalorimetry. Int J Pharm, 104:135-144, 1994.
- Briggner L.-E, Buckton G, Bystrom K, Darcy P. The use of isothermal microcalorimetry in the study of changes in crystallinity induced during the processing of powders. Int J Pharm, 105:125-135, 1994.
- Buckton G. Applications of isothermal microcalorimetry in the pharmaceutical sciences. Thermochimica Acta, 248:117-129, 1995.
- Kawakami K, Numa T, Ida Y. Assessment of amorphous content by microcalorimetry. J Pharm Sci, 91:417-423, 2002.
- Al-Hadithi D, Buckton G, Brocchini S. Quantification of amorphous content in mixed systems: amorphous trehalose with lactose. Thermochimica Acta, 417:193-199, 2004.
- Dilworth SE, Buckton G, Gaisford S, Ramos R, 2004. Approached to determine the enthalpy of crystallisation, and amorphous content, of lactose from isothermal calorimetric data. Int J Pharm, 284:83-94, 2004.
- Ahmed H, Buckton G, Rawlins DA. The use of isothermal microcalorimetry in the study of small degrees of amorphous content of a hydrophobic powder. Int J Pharm, 130:195-201, 1996.
- Ohta M, Tozuka Y, Oguchi T, Yamamoto K. Water vapour adsorption properties of amorphous cefditoren pivoxil evaluated by adsorption isotherms and microcalorimetry. Drug Dev Ind Pharm, 26:643-649, 2000.
- Mackin L, Zamon R, Park JM, Foster K, Opalenik H, Demonte M. Quantification of low levels (< 10%) of amorphous content in micronised active batches using dynamic vapour sorption and isothermal microcalorimetry. Int J Pharm, 231:227-236, 2002.
- Samra RM, BucktonG. The crysatllisation of a model hydrophobic drug (terfenadine) following exposure to humidity and organic vapours. Int J Pharm, 284:53-60, 2004.
- Vemuri NM, Chrzan Z, Cavatur R. Use of isothermal microcalorimetry in pharmaceutical preformulation studies. Part II. Amorphous phase quantification in a predominantly crystalline phase. J Therm Anal Cal, 78:55-62, 2004.
- Sheridan PL, Buckton G, Storey DE. Development of a flow microcalorimetry method for the assessment of surface properties of powders. Pharm Res, 12:1025-1030, 1995.
- Otsuka M, Kato F, Matsuda Y. Physicochemical stability of cimeditine amorphous forms estimated by isothermal microcalorimetry. AAPS PharmaSciTech Technical, 3:1-13, 2002.
- Ramos R, Gaisford S, Buckton G. Calorimetric determination of amorphous content in lactose: A note on the preparation of calibration curves. Int J Pharm, 300:13-21, 2005.
- Buckton G, Darcy P. Assessment of disorder in crystalline powders – a review of analytical techniques and their application. Int J Pharm, 179:141-158, 1999.
- Pikal MJ, Lukes AL, Lang JE, Gaines K. Quantitative Crystallinity determinations for b-Lactam antibiotics by solution calorimetry: correlations with stability. J Pharm Sci, 67:767-772, 1977.
- Hogan SE, Buckton G. The quantification of small degrees of disorder in lactose using solution calorimetry. Int J Pharm, 207:57-64, 2000.
- Harjunen P, Vesa-Pekka L, Koivisto M, Levonen E, Paronen P, Jarvinen K. Determination of amorphous content of lactose by solution calorimetry. Drug Dev Ind Pharm, 30:809-815, 2004.
- Gao D, Rytting JH. Use of solution calorimetry to determine the extent of crystallinity of drugs and excipients. Int J Pharm, 151:183-192, 1997.
- Harjunen P, Lemmo VP, Välisaari J, Lankinen T, Paronen P, Järvinen K. Effects of ethanol to water ratio in feed solution on the crystallinity of spray-dried lactose. Drug Dev Ind Pharm, 28:949-955, 2002.
- Katainen E, Niemelä P, Harjunen P, Suhonen J, Järvinen K. Evaluation of the amorphous content of lactose by solution calorimetry and Raman spectroscopy. Talanta, 68:1-5, 2005.
- Höhne GWH, Hemminger WF, Flammersheim H-J. Applications of differential scanning calorimetry. Springer (New York), 147-244, 2003.
- Gombás Á, Szabó-Révész P, Kata M, Regdon GJ, Eros I. Quantitative determination of crystallinity of a-lactose monohydrate by DSC. J Therm Anal Cal, 68:503-510, 2002.
- Lappalainen M, Pitkänen I, Harjunen P. Quantification of low levels of amorphous content in sucrose by HyperDSC. Int J Pharm, 307:150-155, 2006.
- Hurtta M, Pitkänen I. Quantification of low levels of amorphous content in maltilol. Thermochimica Acta, 419:19-29, 2004.
- Roos Y, Karel M. Plasticising effect of water on thermal behaviour and crystallisation of amorphous food models. J Food Sci, 56:38-43, 1991.
- Hancock BC, Zografi G. The relationship between the glass transition temperature and the water content of amorphous pharmaceutical solids. Pharm Res, 11:471-477, 1994.
- Saunders M, Podluii K, Shergill S, Buckton G, Royall P. The potential of high speed DSC (Hyper-DSC) for the detection and quantification of small amounts of amorphous content in predominantly crystalline samples. Int J Pharm, 274:35-40, 2004.
- Guinot S, Leveiller F. The use of MTDSC to assess the amorphous phase content of a micronised drug substance. Int J Pharm, 192:63-75, 1999.
- Saklatvala R, Royall PG, Craig DQM, 1999. The detection of amorphous material in a nominally crystalline drug using modulated temperature DSC – a case study. Int J Pharm, 192:55-62, 1999.
- Pijpers TFJ, Mathot VBF, Goderis B, Scherrenberg RL, van der Vegte EN. High speed calorimetry for the study of the kinetics of (de)vitrification, crystallisation and melting of macromolecules. Macromolecules, 35:3601-3613, 2002.
- Yoshioka M, Hancock BC, Zografi G. Crystallisation of indomethacin for amorphous state below and above its glass transition temperature. J Pharm Sci, 83:1700-1705, 1994.
- Saleki-Gerhardt A, Ahlneck C, Zografi G. Assessment of disorder in crystalline solids. Int J Pharm, 101:237-247, 1994.
- Fix I, Steffens K-J. Quantifying low amorphous or crystalline amounts of alpha-lactose-monohydrate using X-Ray Powder Diffraction, Near-Infrared Spectroscopy, and Differential Scanning Calorimetry. Drug Dev Ind Pharm, 30:513-523, 2004.




