Ion channel electrophysiology in pharmaceutical research
Posted: 23 May 2007 | | No comments yet
Ion channels are well recognised as targets for a vast range of disease states and conditions. The process of discovering drugs is influenced by the biological confidence in the rationale of the screening approach and the screenability. Various methods have been gathered around the gold standard of manual patch-clamping that account for the higher number of datapoints in functional electrophysiology.
Ion channels are well recognised as targets for a vast range of disease states and conditions. The process of discovering drugs is influenced by the biological confidence in the rationale of the screening approach and the screenability. Various methods have been gathered around the gold standard of manual patch-clamping that account for the higher number of datapoints in functional electrophysiology.
Ion channels are well recognised as targets for a vast range of disease states and conditions. The process of discovering drugs is influenced by the biological confidence in the rationale of the screening approach and the screenability. Various methods have been gathered around the gold standard of manual patch-clamping that account for the higher number of datapoints in functional electrophysiology.
Real UHTS, but non-functional fluorescence or ligand binding methods, were recently added by various automated patch clamp techniques that could partially establish datapoints in a midthroughput range. Since the establishment of reliable high quality data remains crucial in drug discovery and safety assessment, the chronology of screening technologies applied in the discovery remains important. This article provides background information on ion channel electrophysiology, as well as an overview of its current employment in pharmaceutical discovery and drug development.
Ion channels are membrane-bound proteins that allow the passage of ions across impermeable cell membranes. Their most obvious role is to generate action potentials for cardiac and neuronal cells. In addition, they have other extensive functions in cell volume control, differentiation and growth of cells and neurotransmitter release. Structurally, they can be divided into two major families; voltage-gated and ligand-gated ion channels.
Through structural elucidation and sequencing of human and other genomes it became evident that these superfamilies form approximately 60 pore forming subunits, and more than 120 functional units are due to the formation of both homomers and heteromers. Accessory subunits and splice variants are common; implying that the ion channel diversity is enormous. In addition, ion channel functionality is underlying a large array of regulatory proteins (Zambrowicz et al. 2003).
Toxins form natural organisms proved to be invaluable in our understanding of many ion channels (for example, alpha-bungarotoxin in allowing the purification and the cloning of nAChR subunits). Most venoms and natural toxins, inhibitors or activators of ion channels, are highly subtype specific and active with high affinity (for example tetrodotoxin, scorpion toxins, sea anemone and pyrethroids, for voltage sensitive sodium channels, dendrotoxins and apamin for potassium channels). Apart from toxins being leads for future drug discovery, they point to the fact that ion channel drug discovery is able to yield compounds with high specificity and high affinity for individual ion channel populations.
Many diseases are linked with aberrant ion channel function, either due to mutation in ion channel genes themselves, or to mutations in proteins involved in ion channel expression such as transcription factors, membrane targeting or regulation of channel activity. Out of more than 50 described as inherited channelopathies, the most prominent prototypical ion channel genetic disease is certainly cystic fibrosis (CF) due to a mutation in the CFTR gene, showing recessive inheritance. In the context of cardiac safety pharmacology assessment of drugs, LQTS mutations in patients with antiarrhythmic drug-associated arrhythmia are an interesting field of recent investigation in channelopathies (Lehtonen et al. 2007; Physiologie Ulm 2007).
Screening trends
Ion channels are the third most frequent biochemical class of targets for marketed small molecules behind enzymes and G-protein coupled receptors (Hopkins et al. 2002). Drugs acting on ion channels are among the best selling drugs and cover a wide field of indication. Examples are cardiovascular drugs acting primarily on; L-type calcium channels, cardiac type sodium channels, CNS-drugs acting on GABAA receptors (e.g. benzodiazepines) and central nervous sodium channels (e.g. anticonvulsants). Drugs acting at pancreatic ion channels are targeted by antidiabetics and immunosuppressive agents exert their effects, among other mechanisms, via potassium channels (Kv1.3). All these drugs account for sales in the order of 15 billion USD. Discovery screening programmes for these mentioned ion channels continue on a high throughput level.
Moreover, our daily functional screening routine was recently added to by programmes to screen for CNS effects mediated via subtypes of the GABAA channels, the ionotropic subclass of glutamate receptors e.g. AMPAR, NMDAR, neuropathic pain-relevant calcium and sodium channels (e.g. Nav1.8 and 1.9), TRP and ion channels involved in inflammation. The latter screening trend is reflecting advanced knowledge of neurobiology and a heightened perception of the commercial value of neuropathic pain therapeutics. Screening programmes for urinary incontinence, muscular diseases and some forms of cancer represent new trends, including research in nicotinic acetylcholine receptors (nAChRs) that play many critical roles in nervous system function and that have been implicated in a variety of diseases.
In addition to being drug targets, ion channels may mediate unwanted side effects. The discovery of the hERG channel and its underlying role in human torsade de pointes made the isolated hERG channel a pivotal target in non clinical safety screening now being reflected in regulatory guidance for preclinical testing. The hERG channel is one of the potassium channels that contribute to the repolarization of the cardiac action potential and is the target of certain drugs for fatal and lethal arrhythmia (e.g. terfenadine or dofetilide) showing a strong correlation between a block and prolongation of the cardiac action potential (Roden et al. 2005; Redfern et al. 2003). Since further cardiac ion channels may mitigate or increase changes in action potential duration, typical targets for cardiac safety profiling constitute, among others, KvLQT1/minK, further potassium channels and accessory subunits, L-type calcium channels and sodium channels. Action potential studies in isolated cardiac preparations may be used to specifically address organ electrophysiology, to detect proarryhthmic effects and eventually lead to preclinical in vivo methods where ECG parameters are determined. (ICH S7B 2005).
Assay types
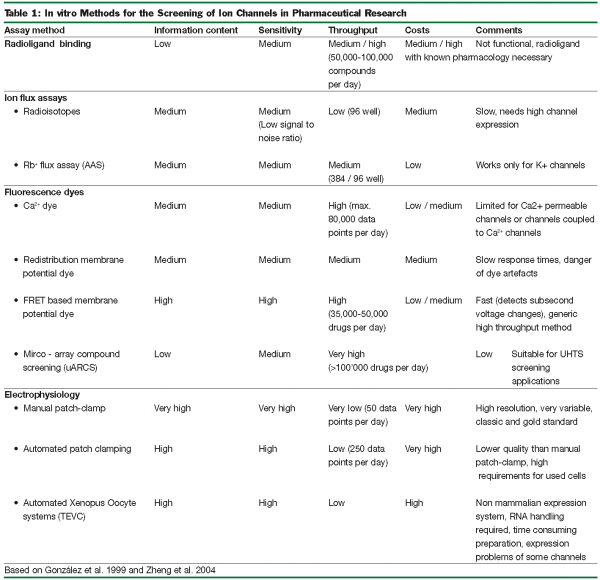
To tackle the ever-increasing number of biochemical compounds to be examined for their effect on ion channels, high throughput systems (HTS) and x (UHTS) are needed. For early screening-assays the test method used has to be rapid, low-cost and should require only a small amount of the test compound. Such systems are less well developed for the target class of ion channels than for other target classes due to technical peculiarities. The following passages provide a brief overview of the commonly used test systems and their advantages and disadvantages will be discussed (Table 1).
In general, ion channel testing methods can be divided into either high-throughput, low-information assays or low-throughput, high-information assays. One example for the high-throughput, low information-type are ligand displacement assays. These systems use a radioactively labelled compound with well known pharmacological activity to occupy a known binding site, subsequently being displaced by the tested compound. A disadvantage is that the effects will remain undetected if the tested compound binds to an uncoupled, weakly allosterically coupled binding site.
Whereas ligand displacement assays require only isolated membranes that contain a high level of the target channel, all further testing methods described below are cell-based. For this purpose, either native cells containing the target protein or overexpression-systems may be used. In overexpression-systems specific problems may arise, as many ion channels are only functional if expressed along with several – partly unknown – subunits or cofactors, or if expressed in a certain stochiometry.
The flux assays are among the most commonly used HTS for ion channels. In these assays, cells are loaded with a detectable ion that mimics the natural ion. The most famous example is Rb+ that permeates through K+ channels with similar properties like K+. Rb+ may then be detected by using atomic absorption spectrometry (AAS) (Terstappen et al. 2004) or scintillation if its radioactive isotope 86Rb+ is used, radioactive tracers for Na+, Ca2+ and Cl- are available. Depending on the channel in these assays, either infux or efflux is measured. Flux assays are robust but loading the cells with the tracer ions is time consuming. Ion flux across the plasma membrane can also be tracked using ion specific fluorescent indicators. Such probes exist for various ions, but their application is almost exclusively limited to Ca2+ measurement. Ca2+ signal may also be used to study sodium channels.
All assays based on fluorescence shifted to HTS after the introduction of the fluorescence imaging plate reader FLIPR using an argon laser to rapidly scan a multi well plate containing dye-loaded cells and a semiconfocal detection method, resulting in excellent sensitivity and high signal to noise ratio.
A much more sensitive method is found in the determination of the plasma membrane potential. This high sensitivity is caused by the high electrical resistance of the biological membranes, which allow small ionic currents to change the membrane potential dramatically. The most common fluorescent probe for this measurement is the oxonol DiBAC4. Oxonols are negatively charged and membrane-permeable. Outside the cell its fluorescence is very weak but upon depolarization it enters the cell and fluorescence yield increases. This redistribution process takes a few minutes and thus, is only suitable to detect changes in the steady-state membrane potential (Burbaum et al.1998). The draw back of this assay type is that, in addition to overexpressed channels, endogenous ion channels contriubue to changes in membrane potential and may therefore render the answer unspecific.
Many effects can not be detected with the slow redistribution method, as many ion channels close their pores very quickly due to channel desensitization or inactivation. Improved, faster assays for HTS detection of membrane potential by fluorescence are based on FRET (Fluorescence Resonance Energy Transfer) (Gonzales & Tsien 1995). For this method, two dyes are loaded into the plasma membrane, a modified voltage sensitive oxonol DiBAC2 used as FRET acceptor and a voltage insensitive coumarin linked phospholipid, which is attached to the outer leaflet of the plasma membrane that acts as FRET donor. During hyperpolarisation, the negatively charged oxonol will be located to the outer leaflet of the plasma membrane resulting in effective FRET (coumarin is quenched, oxonol becomes excited). Depolarisation results in the opposite effect, decreasing FRET effects. This type of assay can be used for a broad variety of ion channels. It is 100 times faster than the redistribution method, very sensitive (1% / mV), has a very high time resolution (10ms, enough to detect an action potential) and can be used as HTS (5000-7000 compounds/day). The FRET assays can be made even more sensitive to screen voltage gated ion channels if they are combined with a Trans Cell Layer Electrical Field Stimulation (TCL-EFS) (Bugianesi et al. 2006).
Patch-clamping versus non-functional methods
Patch-clamp electrophysiology is regarded as the “gold standard” for measuring ion channel activity and pharmacology and is based on analysing membrane properties of a single cell. In the most common “whole cell” conformation, one electrode is in direct contact with the cytoplasm, while the reference electrode is being placed in the extracellular bath. This arrangement allows control of the membrane potential. The resulting high information content is based on the excellent time resolution in the microsecond range and sensitivity in the picoampere range. It is even possible to investigate the properties of a single ion channel molecule and also to provide additional information about voltage dependence, gating behaviour, and rate, as well as use-dependence of compound binding. This universally applicable method is relatively time consuming and requires highly trained and educated operators; facts that can often make manual patch-clamping impractical for primary screenings. To scale up the throughput, many attempts have been made to automate the patch-clamp method. One breakthrough on the way to HTS-patch-clamping was the development of the planar patch-clamp chip.
When using the manual approach, a glass micropipette electrode is moved to an adherent cell using a micromanipulator. The chip based technique works the inverted way. Small holes in the chip surface act as pipette openings, and cells applied to the openings, will eventually form tight gigaohm seals, similar to traditional patch-clamping. During the recent HTS developments it became evident that the full automation of the patch clamp technique was much more difficult than expected. Currently, several automated systems are available (e.g. Sophion’s QPatch) that are shown to increase throughput by 10-100 times compared to manual patch-clamping. Depending on the automated system, fairly untypical acceptance criteria application were suggested in order to achieve increased throughput (e.g. definition of gigaohm seals, single cell paradigms, cell quality) which compromises data reliability. Investments in ideal cell expression systems, dedicated cell handling, operator interventions, liquid preparation and disposables render the datapoint price high compared with non-functional HTS methods. Recently, cost effective semi-automatic devices became available that provide a 3-10 fold speedup compared with conventional handling (e.g. Cellectricon’s Dynaflow, Figure 1).
In our drug discovery routine, UHTS and HTS methods are successfully employed to swiftly generate data for large compound libraries. Subsequent verification of obtained hits is conducted using functional patch-clamp assays. If prevailing coefficients for specificity or sensitivity of non-functional methods are considered to produce an intolerable number of false positive or false negative results; a situation that can not often be accepted for smaller compound collections or compounds that undergo cardiac safety evaluation, patch-clamp is increasingly considered as first line of screening. If high quality data is required, semi-automated or manual patch clamping is the method of choice.
Summarised, patch-clamp provides the highest information content of all screening methods employed, though at a rather low throughput with relatively high costs per data point, which suggests its application is mostly for secondary screening, hit optimisation and ion channel safety assessment (Comley et al. 2003; Zheng et al. 2004).
Achieving specificity with ion channel modulators
A major hurdle in the establishment of drug specificity is the great number of closely related ion channel targets, especially in the potassium channel arena. Furthermore, our understanding of the basis of receptor/channel heterogeneity remains limited. Is heterogeneity coupled to specific physiological functions or does the observed variability of subtypes within a single channel population reflect only Darwinian evolutionary noise? The following passages present the answers to this question for the GABAA receptor field and two examples of our current screening activities are given to outline efforts to achieve ion channel specificity with channel modulators.
Valium’s drawback
Valium, the prototype anxiolytic benzodiazepine invented by Leo Sternbach at Hoffmann-La Roche, reached the market in 1963 (Figure 2). It was the top selling pharmaceutical in the United States from 1969 to 1982, the sales of today still amount to 100 million USD. The drug exerts its therapeutic effects in areas of the limbic system, thalamus, hypothalamus. Valium is a positive allosteric modulator of the GABAA receptor, which mediates fast inhibitory synaptic transmission in the central nervous system. Upon binding of the drug to the benzodiazepine binding site, the GABAA receptor undergoes conformational changes resulting in a higher affinity for the neurotransmitter GABA (Sigel et al. 1997). Despite its wanted anxiolytic profile, Valium is associated with the following side effects; tolerance, sedation, amnesia, depression and alcohol potentiation. The observed adverse effects arise from interactions with other subtypes of GABAA receptors. It is therefore reasonable to search for new drugs displaying a narrower pharmacological profile (at best a unique action) with fewer side effects.
GABAA receptor subtype specificity
The proof for this concept, at least within the GABAA receptor community, was provided independently by researchers from the University of Zurich, Switzerland, and Merck, Sharp and Dohme in Harlow, United Kingdom. Uwe Rudolph & Hanns Möhler constructed transgenic mice expressing GABAA receptor isoforms that are insensitive to the benzodiazepine Valium and investigated the animals for loss of the known Valium effects. It could be demonstrated that the anxiolytic effects are conducted through α2 subunit containing GABAA receptor combinations (Rudolph et al. 1999). Using similar methodologies, Ruth McKernan & Paul Whiting showed that the α1 isoform mediates sedative but not anxiolytic effects (McKernan et al. 2000).
Thus, the pharmacological distinction of GABAA receptor isoforms serves as a promising basis for the development of new therapeutic agents acting only at a specific set of GABAA receptor isoforms mediating neuronal “buttons” for the regulation of major brain processing like learning and memory, sedation, sleep, mood or anxiety (Figure 3). Such compounds are therefore believed to have new therapeutic indications in the field of cognitive enhancers, anesthetics, antidepressants, anticonvulsants and non-sedating anxiolytics.
Stimulants for cognitive processing
Today, almost everybody is talking about Viagra, which already seems to equal the status of a super-drug. This pill is undoubtedly capable of improving personal performance. However, there are now accumulating reports that the future may soon belong to a different pill called a ‘cognitive enhancer’. It is the sort of drug one would swallow if one feels a tired and has to study for an exam the next morning. By stimulating neural activity, this drug should ideally enhance memory, concentration, planning, attention, learning and so on.
GABAA α5 receptors have a relatively restricted distribution being primarily expressed in the hippocampus, a region of the brain important for learning and memory. Although α5 receptors account for less than 5% of the total GABAA receptor population in the brain, in the hippocampus they make up the vast majority of GABAA receptors. Compounds that can reduce the inhibitory effects mediated through GABA-ergic neurotransmission must be either negative modulators, antagonists or channel blockers of the GABAA receptor. Whilst both antagonists and channel blockers will switch-off receptor activity and thereby uncouple signal transmission from presynaptic activity, agents that just negatively modulate GABAA receptor function are believed to sustain general signal flow.
Since negative allosteric modulators of the GABAA receptors are commonly known to display anxiogenic, convulsant and proconvulsant activities, compounds with a decreased potential for these adverse effects have to be developed. This might be achieved by the identification of GABAA receptor subtype selective ligands that exhibit a more specific physiological activity. Such α5 selective negative allosteric modulators of the GABAA receptor promise to be successful candidates in cognitive psychopharmacology; lacking the unwanted side effects associated with activity at other GABAA receptor subtypes, i.e., convulsant and/or anxiogenic effects (Venault et al. 1986).
Subtype specific HTS Screening
The discovery of new ion channel drugs, especially in the ligand-gated ion channel field (due to a severe time lag in signal detection) was thus far hampered by the lack of appropriate HTS screening tools (discovery bottleneck, see section assay types). Radioligand binding studies were long seen as adequate alternative solutions and some institutions therefore relied on this indirect approach, which was potentially the major cause for their failure to establish drug specificity. For reasons which are not discussed here, selective GABAA receptor isoform activity can be achieved only in terms of functional selectivity rather than binding selectivity (personal communication Paul Whiting). The classical whole-cell patch-clamping or the today available robotic electrophysiology workstations are not yet suitable methodologies for true HTS applications.
Based on these facts, the development of a GABAA receptor HTS screening platform was initiated early in 2004, including the internal cloning of stable cell lines expressing the desired GABAA receptor targets. To achieve 384-well HTS format we focused on a FMP-dye based FLIPR technology. The distribution of FMP-dye across the intracellular/extracellular compartments is voltage-dependent. This allows the measurement of changes in the membrane potential, which is sufficiently sensitive to distinguish and quantify small stimulations and inhibitions of GABAA receptor currents (Figure 4). The synchronous analysis of various GABAA receptor subtypes (α1, α2, α5 and others) on cell-based matrix plates allows the HTS detection of isoform selective compounds at the earliest drug development stages.
September 2005’s take-off of the screening platform greatly leveraged our drug discovery programme. Molecules from focused libraries are tested for stimulatory GABAA receptor activity by means of the FLIPR technology. In addition, a maximally diverse subset of compounds is analysed with the newly introduced micro arrayed compound screening (µARCS) assay, ideally suited for uHTS needs (Figure 3). The identified hits include novel specific modulators as well as new agonists of the GABAA receptor, which are subjected to patch-clamping assays for hit verification and further analysis of mode of action (Figure 5).
Safer class III antiarrhythmics
In another discovery programme, electrophysiology screening is employed to design new class III antiarrhythmics lacking adverse reactions, thus establishing more specificity and better therapeutic profiling (Waldhauser et al. 2006). For instance, Amiodarone (Figure 2) is widely used for the treatment of atrial fibrillation, acute myocardial infarction or congestive heart failure (Singh et al. 1996). It targets the HERG potassium channel and thus prolongs the cardiac action potential (Kiehn et al. 1999). Apart from its therapeutic action, the drug displays mitochondrial toxicity.
In order to separate the toxicity function of Amiodarone, analogues were synthesized and screened for minimal mitochondrial toxicity, whilst retaining the blocking effect through the HERG potassium channel (Figure 5). The successful candidates currently undergo chemical refinement for further optimisation of therapeutic specificity; with the ambition of developing Amiodarone derivatives with class III antiarrhythmic activity and lower hepatotoxicity.


Figure 1: The Dynaflow System from Cellectricon enables classical patch-clamping at accelerated throughput and at high quality & accuracy. The recording pipette is moved in front of a microfluidic chip delivering specific drug solutions to a patch-clamped cell. Switching of solution channels occurs in the ms range, ideally suited for the analysis of ligand-gated channels, but also HERG potassium channels in ICH-driven GLP studies.
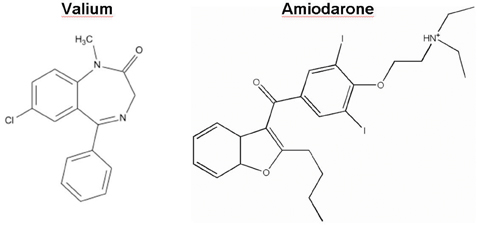

Figure 2: The chemical structures of Valium (diazepam), a positive allosteric modulator of the GABAA receptor, and the class III antiarrhythmic Amiodarone are shown.
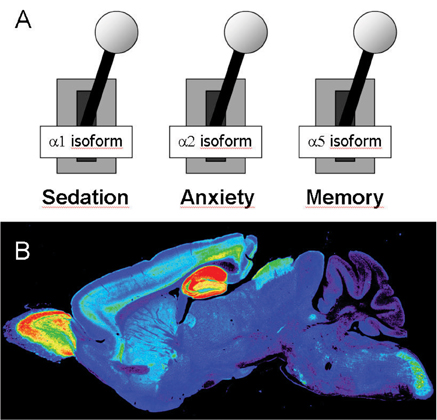

Figure3: (A) The separation of benzodiazepine actions through distinct GABAA receptor subtypes indicates different physiological functions of these receptor isoforms, promoting the design of drugs specifically modulating selected GABAA receptor subtypes. (B) The regional and cellular expression of the a5 subtype in mouse brain is presented (according to Crestani et al. 2002).
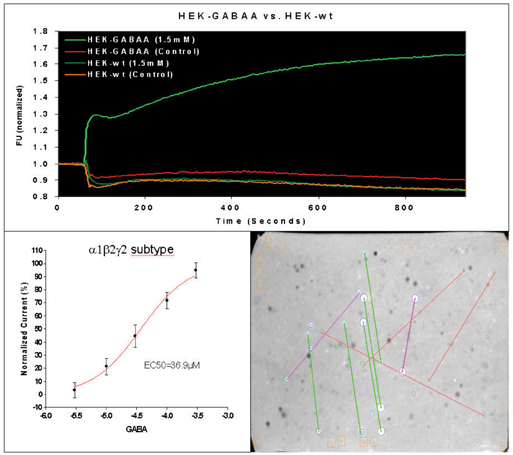

Figure 4: (A) The FLIPR traces of typical measurements with wildtype HEK 293 cells and HEK 293 cells expressing GABAA receptors are presented. The average of baseline before drug or buffer addition (controls) was used for the normalization of traces. (B) The established EC50 value for GABA was 36.9µM. (C) The micro-array compound screening (µARCS) technology is an UHTS assay. Cells are casted into an agarose gel in the presence of the FMP-dye. Then, the agarose gel is brought into contact with an array of drugs spotted on ChemCards (Trademark of Biofocus DPI). A ChemCard holds approximately 5000 substances in duplicate and corresponds to the size of standard micro titer plates. Upon diffusion of drugs into gel, local changes of the membrane potential are caused by agonists and visualized as distinct spots by means of a CCD-based fluorescence reader.
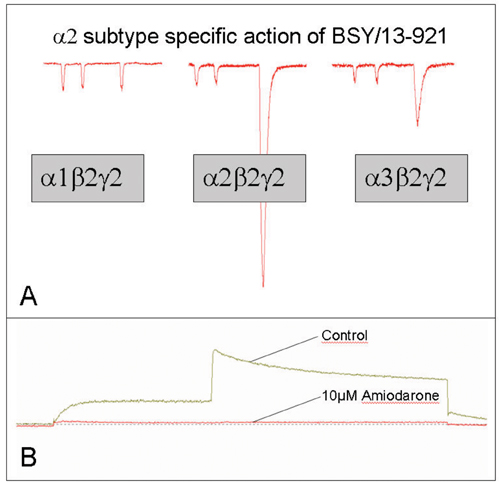

Figure 5: (A) Representative current traces of a subtype specific ligand are outlined. First two submaximal applications of the neurotransmitter GABA (approximately GABA EC5) evoke inward chloride currents. Subsequently, a coapplication of GABA and BSY/13-921 was perfused to patch-clamped cell. In cells expressing the a2b2g2 subtype, a large stimulation of GABA activity was observed, whereas at the other subtypes tested, the stimulatory effect by BSY/13-921 was much less pronounced. (B) The interaction of Amiodarone with the HERG potassium channel is shown. Amiodarone almost completely blocks the HERG channel (according to Waldhauser et al. 2006).

References
- Burbaum JJ, Sigal NH (1997) New technologies for high-throughput screening. Curr. Opin. Chem. Biol. 1: 72-78. Review.
- Bugianesi RM, Augustine PR, Azer K, Dufresne C, Herrington J, Kath GS, McManus OB, Napolitano CS, Rush A, Sachs J, Simpson N, Wismer MK, Kaczorowski GJ, Slaughter RS (2006) A cell-sparing electric field stimulation technique for high-throughput screening of voltage-gated ion channels. Assay Drug Dev. Technol. 4: 21-35.
- Comley J. (2003) Patchers versus Screeners – Divergent Opinion on High Throughput Electrophysiology, Drug discovery World Fall 2003: 47-57.
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Möhler H, Rudolph U (2002) Trace fear conditioning involves hippocampal a5 GABAA receptors. PNAS 99: 8980-8985.
- Gonzalez JE, Tsien RY (1995) Voltage sensing by fluorescence resonance energy transfer in single cells. Biophys J. 69: 1272-1280.
- Gonzalez JE, Oades K, Leychkis Y, Harootunian A, Negulescu PA (1999) Cell-based assays and instrumentation for screening ion-channel targets. Drug Discov. Today. 4: 431-439.
- Hopkins AL, Groom CR (2002) The druggable genome. Nat. Rev. Drug Discov. 2: 95-96. http://physiologie.uni-ulm.de/angewandte-physiologie/Pdf/overview_tabelle_channelopathies.pdf
- ICHS7B The non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals issued as CHMP/ICH/423/02, adopted by CHMP in May 2005.
- Kiehn J, Thomas D, Karle CA, Schols W and Kubler W (1999) Inhibitory effects of the class III antiarrhythmic drug amiodarone on cloned HERG potassium channels. Naunyn Schmiedebergs Arch. Pharmacol. 359:212-219.
- Lehtonen A, Fodstad H, Laitinen-Forsblom P, Toivonen L, Kontula K, Swan H (2007) Further evidence of inherited long QT syndrome gene mutations in antiarrhythmic drug-associated torsades de pointes. Heart Rhythm. 4:603-607.
- McKernan RM, Rosahl TW, Reynolds DS et al. (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor a1 subtype. Nat. Neurosci. 3: 587-592.
- Redfern WS, Carlsson L, Davis AS (2003) Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 58: 32-45.
- Roden DM and Viswanathan PC (2005) Genetics of acquired long QT syndrome. Journal of Clinical Investigation. 155: 2025-2032.
- Rudolph U, Crestani F, Benke D et al. (1999) Benzodiazepine actions mediated by specific g-aminobutyric acid type A receptor subtypes. Nature 401: 796-800.
- Sigel E and Buhr A (1997) The benzodiazepine binding site on GABAA receptors. Trends Pharmacol. Sci. 18:425-429. Review.
- Singh BN (1996) Antiarrhythmic actions of amiodarone: a profile of a paradoxical agent. Am. J. Cardiol. 78:41-53.
- Terstappen GC. (2004) Nonradioactive rubidium ion efflux assay and its applications in drug discovery and development. Assay Drug Dev. Technol. 2: 553-559. Review.
- Venault P, Chapouthier G, Prado de Carvalho L, Simiand J, Morre M, Dodd RH, Rossier J (1986) Benzodiazepine impairs and b-carboline enhances performance in learning and memory tasks. Nature 321: 864-866.
- Waldhauser KM, Török M, Ha HR, Thomet U, Konrad D, Bigler L, Follath F, Krähenbühl S (2006) Hepatotoxicity and pharmacological effect of amiodarone and amiodarone analogues. J. Pharmacol. Exp. Ther. 319: 1413-1423.
- Wood C, Williams C and Waldron GJ (2004) Patch clamping by numbers, DDT 9: 434-441.
- Zambrowicz BP, Sands AT (2003) Knockout model the 100 best selling drugs – will they model the next 100? Nat. Rev. Drug Discov. 2: 38-50.
- Zheng W, Spencer RH, Kiss L (2004) High throughput assay technologies for ion channel drug discovery. Assay Drug Dev. Technol. 2004 2: 543-552. Review.
Simon Hebeisen
Simon Hebeisen studied biology at the University of Karlsruhe, Germany. In 2004, he got his PhD in physiology at the University of Aachen, producing a thesis which was honoured by the University foundation council. His major interest field was the study and characterization of various chloride channels. He joined bSys GmbH in 2006 as Head Electrophysiologist.
Prior to this, he has been a project leader at the University of Tübingen, focussing on the establishment of HTS technologies for the investigation of ion channels. He has extensive expertise in ion channel CRO activities and was engaged in numerous ion channel drug development projects spanning from non-sedative anxiolytics to antidiabetics and immuno-suppressive agents.
Urs Thomet
Urs Thomet previously held an academic appointment in neuropharmacology at the Institute of Pharmacology and Toxicology with the University of Zurich; working as part of the development of new CNS (Central Nervous System) drugs.
He was also a study director responsible for cardiac in vitro electrophysiology in the field of preclinical safety evaluation at RCC Ltd in Itingen, a leading contract research company. His initial industrial experience was obtained in Basle at Ciba SC where he held a senior scientist position in chemistry. At the University of Berne he completed his PhD thesis in neuroscience on the search for novel drug candidates with potential indications in Alzheimer’s, epilepsy, anxiety, and sleep disorders (2000).
In 1999, together with the research groups of Prof. Erwin Sigel, Dr. Robert Dodd and Prof. Werner Sieghart, he filed two patent applications covering two new classes of breakthrough CNS molecules. He graduated in physical chemistry in 1996. Today, Urs Thomet is Chief Scientific Officer of bSys GmbH.
Daniel Konrad
Daniel Konrad has served as CEO of bSys GmbH since its conception in 1997; he is co-founder and member of the executive board of bSys GmbH. His achievements for the company were honoured in 2004 with the selection of bSys´s team as “New Entrepreneurs in Technology and Science” by the ETH Zurich and the Gebert Rüf Foundation.
He graduated in medicine in 1993 at the University of Basle, Switzerland. Daniel Konrad has a track record in pre-clinical development both for small molecules and biologics, and also served as Project Leader for Medical Devices (RCC Ltd.) His scientific work was published in various medical and scientific journals and he was invited as a speaker to numerous conferences. Since 2007 he has been appointed as President of the Basle Ion Channel Platform (BASICP).



