One- & two-year survival rates of 94% and 88% announced from Phase 1b trial of investigational PD-1 checkpoint inhibitor nivolumab and yervoy® (ipilimumab) in advanced melanoma
Posted: 3 June 2014 | | 1 comment
Bristol-Myers Squibb Company announced follow up results from Study -004, a multi-arm Phase 1b dose-ranging trial evaluating the safety and activity of the combination regimen of nivolumab…


Bristol-Myers Squibb Company (NYSE: BMY) today announced follow up results from Study -004, a multi-arm Phase 1b dose-ranging trial evaluating the safety and activity of the combination regimen of nivolumab, an investigational PD-1 immune checkpoint inhibitor, and Yervoy® (ipilimumab) given either concurrently or sequentially in patients with advanced melanoma (n=127). After an additional year of follow up of the cohort that received the concurrent combination regimen of nivolumab 1 mg/kg plus Yervoy 3mg/kg (n=17), the one-year overall survival (OS) rate was 94% and the two-year OS rate was 88%. These are the doses used in the ongoing Phase 2 and Phase 3 trials, CheckMate -069 and -067. No new safety signals were reported in the concurrent combination cohorts with additional follow up (n=53) and grade 3-4 treatment-related adverse events (AEs) occurred in 62% of patients. The most common were asymptomatic increases in lipase (15%), ALT (12%) and AST (11%). These data will be presented today at the 50th Annual Meeting of the American Society of Clinical Oncology (ASCO) and featured during an ASCO press briefing at 8 a.m. CDT (Abstract # LBA9003).
“The treatment of advanced melanoma has changed dramatically in the last few years, but there continues to be a need to increase the number of patients who experience a long-term survival benefit,” said Dr. Mario Sznol, Yale University School of Medicine and Yale Cancer Center, presenter of the results. “While these are Phase 1b data, the duration of response and one- and two-year survival rates observed with the combination regimen of nivolumab and Yervoy are very encouraging and support the rationale for the ongoing, late stage trials of this combination regimen.”
“The science of immuno-oncology – harnessing the patient’s immune system to treat cancer – is rapidly evolving,” said Michael Giordano, senior vice president, Head of Development, Oncology & Immunology at Bristol-Myers Squibb. “These results are the most advanced data set to date evaluating the potential of combining immune checkpoint inhibitors. As leaders in the field, they reinforce our aspiration that combining immunotherapies may be foundational and may have the potential to change the standard of care by transforming survival expectations.”
Results from Phase 1b Combination Regimen (Study -004)
Study 004 is a dose-ranging Phase 1 study (n=127) evaluating the safety, antitumor activity and pharmacokinetics of the combination regimen of nivolumab and Yervoy given concurrently or sequentially in patients with advanced melanoma. Prior to enrollment, patients could have received up to three systemic therapies.
In the concurrent regimen cohort (n=53), eligible patients received nivolumab and Yervoy every three weeks for four doses, followed by nivolumab alone every three weeks for four doses. This concurrent combination regimen treatment was subsequently continued every 12 weeks for up to eight doses. Cohorts of a maximum of 17 patients per dose level were enrolled (nivolumab 0.3 mg/kg + Yervoy 3 mg/kg [n=14]; nivolumab 1 mg/kg + Yervoy 3 mg/kg [n=17]; nivolumab 3 mg/kg + Yervoy at an investigational dose of 1 mg/kg [n=16]; nivolumab 3 mg/kg + Yervoy 3 mg/kg [n=6]). In an expansion cohort (n=41), eligible patients received the concurrent combination regimen of nivolumab 1 mg/kg and Yervoy 3 mg/kg every three weeks for four doses, followed by nivolumab alone at 3 mg/kg every two weeks until progression, which is the same schedule utilized in the ongoing Phase 2 and Phase 3 trials. In the sequenced regimen cohort (n=33), patients previously treated with Yervoy received nivolumab alone at 1 mg/kg or 3 mg/kg every two weeks.
Results from this trial were first published in the New England Journal of Medicine and presented at ASCO in 2013. The updated data, including those shown below, are based on a median follow up of 22 months and reflect an additional year of follow up from patients initially enrolled in the trial.
Efficacy Summary: Concurrent and Sequenced Cohorts
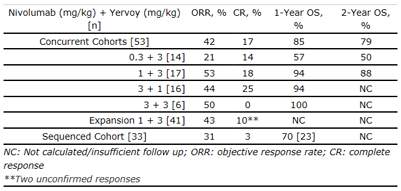

Responses were observed regardless of BRAF mutational status or PD-L1 expression.
No new safety signals were reported with additional follow up. Grade 3-4 treatment-related AEs occurred in 62% of patients in the concurrent cohorts, managed with standard algorithms. The most common were asymptomatic increases in lipase (15%), ALT (12%) and AST (11%). Twenty-two patients (23%) discontinued treatment due to related AEs. There was one drug-related death due to fatal multi-organ failure following an initial event of colitis.




I want to buy Nivolumab and Ipilimumab goods ,now I am in Los angels, my freind will dried, I want to buy the goods ,tell me your company address or shop address I will buy ,my freind is Ca girl,bored in 1989.now wait to dried, please help me,