RS coupled to separations
Posted: 24 March 2006 | | No comments yet
Currently, Raman spectroscopy (RS) is rapidly becoming a mature analytical technique in the pharmaceutical world. Thus far, it is used almost exclusively in a stand-alone mode, since coupling with liquid separation methods hardly seemed realistic in practice. However, as outlined in this article, recent developments are quite promising and such combinations of techniques have a distinct potential.
Currently, Raman spectroscopy (RS) is rapidly becoming a mature analytical technique in the pharmaceutical world. Thus far, it is used almost exclusively in a stand-alone mode, since coupling with liquid separation methods hardly seemed realistic in practice. However, as outlined in this article, recent developments are quite promising and such combinations of techniques have a distinct potential.
Currently, Raman spectroscopy (RS) is rapidly becoming a mature analytical technique in the pharmaceutical world. Thus far, it is used almost exclusively in a stand-alone mode, since coupling with liquid separation methods hardly seemed realistic in practice. However, as outlined in this article, recent developments are quite promising and such combinations of techniques have a distinct potential.
Raman spectroscopy is a mode of vibrational spectroscopy based on the inelastic scattering of (laser) light. It provides detailed molecular structure information like infrared spectroscopy, but is applicable to aqueous samples since water – fortunately – is a very poor Raman scatterer. Quite relevant is the possibility to study materials in situ such as pharmaceutical tablets inside polymer containers1. Furthermore, information on physical structure such as polymorphism can be obtained2. Recently, the US-FDA started the Process Analytical Technology (PAT) initiative in which RS can be successfully applied as a process analyser or process analytical chemistry tool3. A review on this topic appeared in European Pharmaceutical Review, Issue 1 20054. RS is gaining popularity in the pharmaceutical field because sample handling is minimal. In general, there is a strong difference in relative scattering intensity of packaging materials and excipients compared to the active pharmaceutical ingredient (API) so that the latter can be observed directly. Furthermore, Raman instrumentation and sophisticated spectral analysis software such as principal component analysis (PCA) and partial least squares (PLS) are becoming increasingly user-friendly and no longer require highly trained operators.
Nevertheless, RS is not yet involved routinely in all fields where it has a distinct potential. This is due to the inherent drawback of conventional, ‘normal’ RS: inelastic scattering suffers from an extremely low molecular cross section, i.e. it is a very improbable process. Typically only 1 out of 107 photons of excitation light will be scattered inelastically. As a result, there is a continuing struggle with fluorescence background (from the analyte itself or from impurities), which tends to be much stronger and frequently overwhelms the Raman spectrum, even if those impurities are present at much lower concentrations.
Both scatter and fluorescence intensities are dependent on the wavelength (λ) of the laser excitation light. The former increases with λ-4, so the relative intensities at 1000 nm, 500 nm and 250 nm would be 1 : 16 : 256. Unfortunately, upon shortening λ, the fluorescence background often increases as well (although that is not the case for excitation below 260 nm, as will be highlighted below). This explains why, in pharmaceutical practice, either visible or near-infrared lasers are used (up to typically 785 nm) in a dispersive spectrometer setup with a CCD detector, or near infrared lasers (1064 nm) in an interferometer-based system. Apparently, the fact that fluorescence hardly interferes at such long wavelengths outweighs the λ-4 disadvantage.
The limited sensitivity of RS is usually not a problem in the case of pure substances, but it has hampered its breakthrough as a detection and identification technique for liquid separation methods such as liquid chromatography (LC) and capillary electrophoresis (CE). However, in recent years significant progress has been achieved in those areas, and that will be the main subject of the present paper5. Not only will normal RS be considered, but also advanced modes such as resonance RS (RRS), surface-enhanced RS (SERS) and their combination, SERRS. In these applications, RS is mainly used for identification purposes; it should be applied in combination with (UV) absorption detection if quantification is aimed at as well. Both on-line and at-line coupling modes with LC and CE will be considered.
Raman coupled to liquid chromatography
LC–RS
With normal RS, compounds can only be detected at relatively high concentrations, often exceeding the concentrations dealt with in ‘real-life’ LC separations. Typical detection limits (LODs) are in the 0.5–1 mg/ml range6-8 illustrating the challenge of developing LC–RS into a practical method. Options for instrumental improvement include the laser wavelength, the excitation power and the measurement time. Since in RS there is no absorption of laser light by the analyte, high laser powers can be used without saturation effects. For instance, Steinert et al.9 used laser powers up to 8 W (whereas usually only milliWatts of laserpower are applied) and reported LODs of 1 µg/ml for a mixture of m- and p-xylene. A more attractive option is to apply long integration times or signal averaging, but in an on-line flow detection system this will be limited to a few seconds. Longer integration times can be used in stopped-flow experiments (as commonly applied in LC–NMR) and in the at-line mode where spectra are recorded after deposition on a moving substrate.
A very simple illustration of the latter approach was presented by Zhang et al.10,11 who used a drop coating deposition Raman (DCDR) technique for LC fractions. Through solvent evaporation and longer signal integration times better detection limits were obtained: good normal RS spectra were obtained from fractions containing 20 µM human insulin or 3 µM bovine insulin following deposition onto a Teflon-coated stainless-steel substrate.
LC–Liquid-Core Waveguide-RS
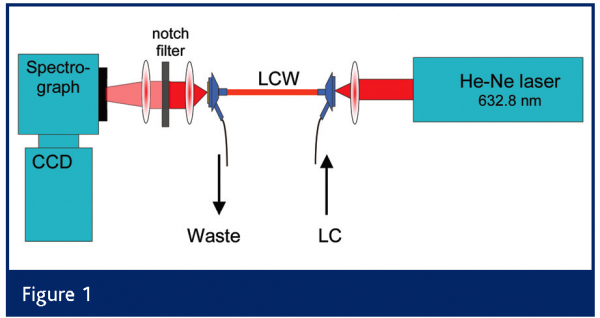
A new and more viable approach to improve analyte detectability in LC–RS is to increase the optical path length without increasing the detector cell volume (which would affect the chromatographic resolution). This can be achieved by means of a liquid-core waveguide (LCW), a narrow tube made of a special plastic material with a refractive index (RI) lower than that of the core liquid, i.e. the eluent12. Light focused into the capillary tube will hit the wall at an angle larger than the critical angle and will be totally internally reflected. As a result, both the laser excitation light and a large fraction of the Raman scattered light will be captured and can travel over large distances inside an LCW. Some ten years ago a material (Teflon AF2400) became available with an RI as low as 1.29, lower than that of water (RI = 1.33) and other common reversed-phase solvents13. Using such an LCW as LC detector cell, an up to 500-fold signal enhancement can be achieved compared to a regular flow cell14.
Various configurations have been explored in LC–LCW-RS15–18; we used a ‘forward’ configuration (see Fig. 1). LCWs with small i.d. (typically 50–200 µm) were used to create detector cells of 0.3–1 m length, while maintaining internal volumes compatible with conventional15,16 or microbore17,18 LC. The maximum length of an LCW is also determined by the transparency of the eluent at the laser wavelength: the vibrational overtone absorption by water is significant for lasers in the far-red and especially in the near-infrared range16. A further improvement of the detectability was realised in our laboratory by using (on-line) analyte enrichment techniques such as large-volume injection, an option frequently overlooked by spectroscopists focusing on detection aspects. We used 500-µl large-volume injection, which resulted in LODs in the 10–100 µg/ml range, a 35-fold improvement compared to standard 10-µl injections12.
It should be realised that the LCW approach does not solve the eluent background and interference problems, since those signals also ‘benefit’ from the longer optical path length. Organic modifiers in the eluent are often strong Raman scatterers and may overwhelm the analyte spectrum16 if not properly corrected for using advanced chemometric approaches. Recently, two eluent-background subtraction methods were reported19,20, resulting in analyte spectra that were highly correlated with the reference spectra.
LC–resonance Raman spectroscopy
A completely different approach to enhance the Raman signal intensity is based on resonance excitation; employing a laser wavelength that overlaps with an electronic absorption band of the molecule. Resonance Raman spectra are typically 102–106 times more intense than normal Raman spectra21. The enhancement affects only the analyte vibrations that couple to the chromophoric group involved in the electronic transition, which is why RRS spectra are often relatively simple: vibrations of a different symmetry and vibrations not connected with the chromophore remain weak. Furthermore, the method provides extra selectivity, since most solvents and matrix materials will be off-resonance and their Raman signals will consequently not be enhanced.
Until the early 1990s, LC–RRS was carried out exclusively in the visible range and background fluorescence was often a major problem. Since then a substantial improvement has been achieved by moving to the UV region. This broadened the applicability range since most analytes show substantial absorptivity in the deep-UV region (<260 nm) and are therefore prone to resonance enhancement. Quite surprisingly, as was first reported by the Asher group21, fluorescence interference is minimal if excitation wavelengths below 260 nm are used (and Raman signals are detected in the 260–285 nm wavelength region or shorter). Our group22 used a continuous-wave frequency-doubled argon laser at 244 nm to perform LC–UV-RRS. In a worst-case approach, a test mixture of fluorene, phenanthrene, fluoranthene and pyrene —which all display intense fluorescence — was separated and could be distinguished on the basis of their RRS spectra. By using routine trace-enrichment techniques, LODs were as low as 50–200 ng/ml and further improvements were achieved by using larger injection volumes and chromatographic stacking. It should be noted that LCWs are not appropriate for RRS due to strong absorption by the analyte at longer path lengths.
LC coupled at-line to surface-enhanced Raman
Another well-known and widely applied technique to obtain intense Raman signals is based on surface enhancement23-25. Suitable SE(R)RS substrates usually have a metallic fine-structure (mainly silver or gold, often as a colloidal suspension) with dimensions of the order of nanometers.
SE(R)RS is most easily coupled to LC in the at-line mode; our group used a spray-jet interface to deposit the LC effluent on a moving TLC plate while simultaneously evaporating the solvent26-28. A splitter had to be used to reduce the LC flow. The entire chromatogram of separated dyes was stored as a trace and small aliquots of silver colloid were manually added. This approach worked well, not only with volatile buffer salts and additives, but also in ion-pair (gradient) LC. Identification limits were in the 50–250-ng/ml range for the cationic dyes26,27, but 100-fold higher for the anionic dyes28.
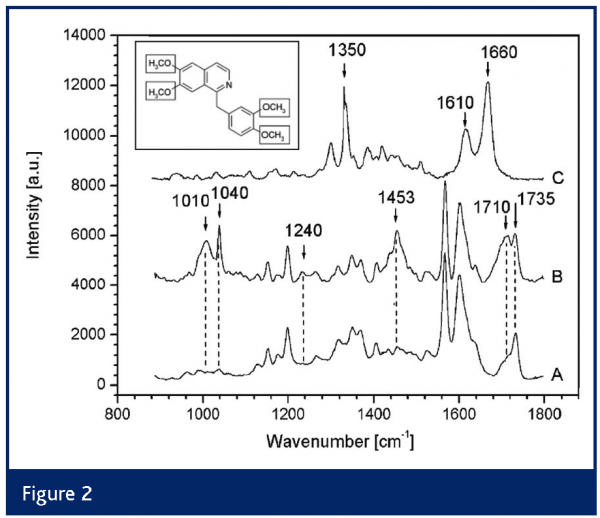
Recently, two real-life applications of at-line LC–SERS were reported by Schneider et al.29,30. Several LC fractions were collected in the wells of a microtiter plate containing a gelatin-stabilised silver halide dispersion. Subsequently, the SERS-active substrate was formed in situ by irradiation with the laser of the Raman microscope. The technique was used to identify the active ingredient in several illicit drugs exhibits (Figure 2). For cocaine-HCl and procaine the LODs were 1–2 µg/well plate (i.e., 100–200 µg/ml), whereas good-quality spectra were recorded of desmethylpapaverine at 75 ng/well plate.
Raman coupled to capillary electrophoresis
Raman detection would be fully compatible with the aqueous buffers typically used in CE, but the optical pathlength problem is even more severe than in LC. Whereas in conventional-size LC path lengths are typically a few mm, in CE 75 μm is a very common diameter. LCWs would have too large an internal volume and are therefore incompatible with CE, but RRS and SERRS are suitable signal enhancement approaches.
CE–RRS
As far back as 1988 the group of Morris and coworkers coupled CE with Raman spectroscopy. Using resonance excitation in the visible range, LODs of 2.5 µM were reported for the model dye compounds methyl red and methyl orange31, about three orders of magnitude better than with normal RS detection32. To improve the LODs of normal RS through preconcentration, the authors used field-amplified injection and/or isotachophoresis (ITP)33,34.
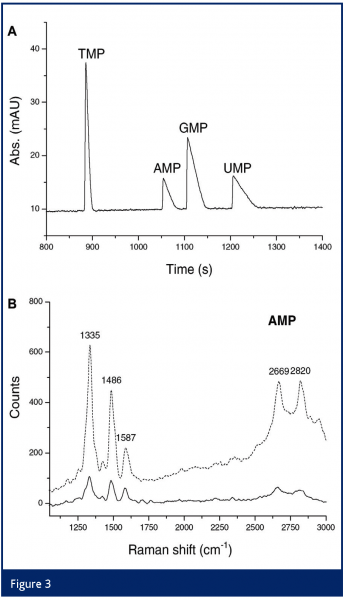
The number of publications devoted to CE–RRS is still modest, probably due to the limited number of compounds that absorb in the visible and the accompanying fluorescence background problem. Again, the deep UV region is promising: we recorded UV-RRS spectra of LC-separated nucleotides on-line at both 244- and 257-nm laser excitation; the highest intensities were obtained for AMP (see Figure 3)35. By using sample stacking, LODs of 0.3 –3 µM were obtained (25–200 µM at the detection window).
CE coupled at-line to SE(R)RS
As with LC, SE(R)RS is most easily coupled to CE in the at-line mode. As for the at-line coupling of SE(R)RS, CE has a brighter future than LC because of its extremely low flow rates (nl/min) and the small analyte spots to be expected after deposition —dimensions which should be fully compatible with Raman microscopy. The problem in CE is to construct an interface that guarantees electrical contact during deposition36,37. Recently, a more robust interface with a stainless steel needle was developed in our group38.
Various SERS-active substrates have been compared: glass plates coated with silver particles36, silver-coated gold particles37, etched silver foil39, vapour-deposited silver film39 and also a TLC plate on which silver colloid and additives were added to the separated analyte spots40. The latter substrate has favourable water-sorption properties and provided the best results, but requires manual addition of the colloid. Cationic dyes were separated and their SERRS spectra were recorded after deposition on various substrates. The spectra were largely independent of the nature of the substrates and although the dyes were structurally very similar they could easily be distinguished.
A bright future
Following its success in the field of stand-alone applications, Raman spectroscopy has the potential to become an important detection method for liquid separation techniques. In our opinion, conventional ‘normal’ RS will never become the preferred method for trace-analysis – the LODs are simply too poor – but it will have a number of pharmaceutical applications, especially if the sensitivity is further improved. Liquid-core waveguides constitute a major improvement, especially if chemometric tools can help to accurately subtract background contributions. RS has a great potential for identifying isomers that are difficult to distinguish using LC–mass spectrometry, for instance in the case of carbohydrates. Another important application regards conformational studies for biopharmaceuticals, where RS can for instance be used to verify the secondary and tertiary structures of proteins.
The future of UV-Resonance RS as a detection method is brighter owing to the added selectivity, and since the detection limits are typically some three orders of magnitude better than with normal RS. Since only vibrations that couple to the chromophoric group are excited, UV-RRS can, for example, be used to study the nature of specific parts of large (bio)molecules. UV-RRS is a more suitable detection method for trace analysis, provided it is combined with (on-line) analyte enrichment techniques such as solid-phase extraction (SPE). The necessary UV lasers, such as frequency-doubled Ar lasers, are nowadays robust and require little maintenance. Several manufacturers offer Raman instruments suitable for the deep-UV.
Surface-enhanced (R)RS is a very sensitive technique (single-molecule detection has been reported) and both LC–SE(R)RS and CE–SE(R)RS will be most welcome in the pharmaceutical laboratory, especially since there is a growing pressure from regulators to elucidate structures of low concentration of impurities. However, research in this area is still in the exploratory stage: in order to make SE(R)RS generally applicable as an at-line detection technique, both suitable deposition interfaces and robust SER(R)S substrates should become commercially available. Being able to store the deposited separations for future studies would be an advantage. If these criteria are met, SER(R)S coupled to liquid separations will be able to provide detailed structural information on the impurities at a very favourable level of sensitivity.



References
- R.L. McCreery, A.J. Horn, J. Spencer, E. Jefferson, 1998. Noninvasive identification of materials inside USP vials with Raman spectroscopy and a Raman spectral library. J. Pharm. Sci. 87: 1-8.
- P. van Hoof, R. Lammers, R. v. Puijenbroek, M. van der Schans, E. Kellenbach, 2002. Polymorphism of the CNS active drug Org 13011: the application of high temperature analysis to detect new polymorphs. Int. J. Pharm. 238: 215-228.
- http://www.fda.gov/cder/OPS/PAT.htm (2006)
- J. Rantanen, 2006. Raman spectroscopy: a process analytical tool. Eur. Pharm. Rev. 2006: 53-57.
- R.J. Dijkstra, F. Ariese, C. Gooijer, U.A.Th. Brinkman, 2005. Raman spectroscopy as a detection method for liquid-separation techniques. TrAC 24: 304-323.
- S.D. Cooper, M.M. Robson, D.N. Batchelder, K.D. Bartle, 1997. Development of a universal Raman detector for microchromatography. Chromatographia 44: 257-262.
- H.G.M. Edwards, A.F. Johnson, I.R. Lewis, 1993. Fourier transform Raman spectroscopic detector system for the analysis of polymers by gel permeation chromatography. J. Raman Spectrosc. 24: 435-441.
- T.D.N. Hong, M. Jouan, N.Q. Dao, M. Bouraly, F. Mantisi, 1996. Coupling of high-performance liquid chromatography with Raman spectrometry. J. Chromatogr. A 743: 323-327.
- R. Steinert, H. Bettermann, K. Kleinermanns, 1997. Identification of xylene isomers in high-pressure liquid chromatography eluates by Raman spectroscopy. Appl. Spectrosc. 51: 1644-1647.
- D.M. Zhang, Y. Xie, M.F. Mrozek, C. Ortiz, V.J. Davisson, D. Ben Amotz, 2003. Raman detection of proteomic analytes. Anal. Chem. 75: 5703-5709.
- C. Ortiz, D.M. Zhang, Y. Xie, V.J. Davisson, D. Ben Amotz, 2004. Identi?cation of insulin variants using Raman spectroscopy. Anal. Biochem. 332: 245-252.
- T. Dallas, P.K. Dasgupta, 2004. Light at the end of the tunnel: recent analytical applications of liquid core waveguides. TrAC 23: 385-392.
- R. Altkorn, I. Koev, R.P. Van Duyne, M. Litorja, 1997. Low-loss liquid-core optical fiber for low-refractive-index liquids: fabrication, characterization, and application in Raman spectroscopy. Appl. Opt. 36: 8992-8998.
- M.J. Pelletier, R. Altkorn, 2001. Raman sensitivity enhancement for aqueous protein samples using a liquid core optical-fiber cell. Anal. Chem. 73: 1393-1397.
- R.J. Dijkstra, A.N. Bader, G.Ph. Hoornweg, U.A.Th. Brinkman, C. Gooijer, 1999. On-line coupling of column liquid chromatography and Raman spectroscopy using a liquid core waveguide. Anal. Chem. 71: 4575-4579.
- R.J. Dijkstra, G.J. Slooten, A. Stortelder, J.B. Buijs, F. Ariese, U.A.Th. Brinkman, C. Gooijer, 2001. Liquid-core waveguide technology for coupling column liquid chromatography and Raman spectroscopy. J. Chromatogr. A 918: 25-36.
- B.J. Marquardt, P.G. Vahey, R.E. Synovec, L.W. Burgess, 1999. A Raman waveguide detector for liquid chromatography. Anal. Chem. 71: 4808-4814.
- B.J. Marquardt, K.P. Turney, L.W. Burgess, 1999. Raman waveguide detector for low analyte concentrations in liquid samples. Proc. Soc. Photo-Opt. Inst. 3860: 239-249.
- R.J. Dijkstra, H.F.M. Boelens, J.A. Westerhuis, F. Ariese, U.A.Th. Brinkman, C. Gooijer, 2004. Hyphenation of column liquid chromatography and Raman spectroscopy via a liquid-core waveguide: chemometrical elimination of spectral eluent background. Anal. Chim. Acta 2004: 129-136.
- B.K. Dable, B.J. Marquardt, K.S. Booksh, 2005. Rapid multivariate curve resolution applied to near real-time process monitoring with HPLC/Raman data. Anal. Chim. Acta 544: 71-81.
- S.A. Asher, 1993. UV resonance Raman spectroscopy for analytical, physical, and biophysical chemistry. Anal. Chem. 65: A59-A66.
- R.J. Dijkstra, C.T. Martha, F. Ariese, U.A.Th. Brinkman, C. Gooijer, 2001. On-line identification method in column liquid chromatography: UV resonance Raman spectroscopy. Anal. Chem. 73: 4977-4982.
- M. Moskovits, 1985. Surface-enhanced spectroscopy. Rev. Mod. Phys. 57: 783-826.
- A. Otto, in M. Cardona, G. Güntherodt (Eds.), Light Scattering in Solids IV. Electronic Scattering, Spin Effects, SERS and Morphic Effects, Springer, Berlin, Germany, 1984, p. 289.
- Z. Q. Tian, 2000. Surface-enhanced Raman spectroscopy; its present status. Int. J. Vib. Spect. 4: 2-14.
- G.W. Somsen, S.K. Coulter, C. Gooijer, N.H. Velthorst, U.A.Th. Brinkman, 1997. Coupling of column liquid chromatography and surface-enhanced resonance Raman spectroscopy via a thin-layer chromatographic plate. Anal. Chim. Acta 349: 189-197.
- R.M. Seifar, R.J. Dijkstra, U.A.Th. Brinkman, C. Gooijer, 1999. At-line coupling of surface-enhanced resonance Raman spectroscopy and reverse-phase ion-pair chromatography. Anal. Commun. 36: 273-276.
- R.M. Seifar, M.A.F. Altelaar, R.J. Dijkstra, F. Ariese, U.A.Th. Brinkman, C. Gooijer, 2000. Surface-enhanced resonance Raman spectroscopy as an identification tool in column liquid chromatography. Anal. Chem. 72: 5718-5724.
- B. Sagmuller, B. Schwarze, G. Brehm, G. Trachta, S. Schneider, 2003. Identification of illicit drugs by a combination of liquid chromatography and surface-enhanced Raman scattering spectroscopy. J. Mol. Struct. 661-662: 279-290.
- G. Trachta, B. Schwarze, B. Sägmuller, G. Brehm, S. Schneider, 2004. Combination of high-performance liquid chromatography and SERS detection applied to the analysis of drugs in human blood and urine. J. Mol. Struct. 693: 175-185.
- C.-Y. Chen, M.D. Morris, 1988. Raman spectroscopic detection system for capillary zone electrophoresis. Appl. Spectrosc. 42: 515-518.
- C.-Y. Chen, M.D. Morris, 1991. Online multichannel Raman-spectroscopic detection system for capillary zone electrophoresis. J. Chromatogr. 540: 355-363.
- W.K. Kowalchyk, P.A. Walker, M.D. Morris, 1995. Rapid normal Raman-spectroscopy of sub-ppm oxy-anion solutions – the role of electrophoretic preconcentration. Appl. Spectrosc. 49: 1183-1188.
- P.A. Walker, M.D. Morris, M.A. Burns, B.N. Johnson, 1998. Isotachophoretic separations on a microchip. Normal Raman spectroscopy detection Anal. Chem. 70: 3766-3769.
- R.J. Dijkstra, E.V. Efremov, F. Ariese, U.A.Th. Brinkman, C. Gooijer, 2003. Capillary electrophoresis coupled on-line with ultraviolet resonance Raman spectroscopy. Anal. Chem. 75: 5697-5702.
- G.L. Devault, M.J. Sepaniak, 2001. Spatially focused deposition of capillary electrophoresis effluent onto surface-enhanced Raman-active substrates for off-column spectroscopy. Electrophoresis 22: 2303-2311.
- L. He, M.J. Natan, C.D. Keating, 2000. Surface-enhanced Raman scattering: A structure specific detection method for capillary electrophoresis. Anal. Chem. 72: 5348-5355.
- D. Arráez Román, E. Efremov, F. Ariese, A. Segura Carretero, C. Gooijer, 2005. Interfacing capillary electrophoresis and surface-enhanced resonance Raman spectroscopy for the determination of dye compounds. Anal. Bioanal. Chem. 382: 180-185.
- R.J. Dijkstra, A. Gerssen, E.V. Efremov, F. Ariese, U.A.Th. Brinkman, C. Gooijer, 2004. Substrates for the at-line coupling of capillary electrophoresis and surface-enhanced Raman spectroscopy. Anal. Chim. Acta 508: 127-134.
- R.M. Seifar, R.J. Dijkstra, A. Gerssen, F. Ariese, U.A.Th. Brinkman, C. Gooijer, 2002. At-line coupling of capillary electrophoresis and surface-enhanced resonance Raman spectroscopy. J. Sep. Sci. 25: 814-818.






