The future of process design
Posted: 20 May 2005 | | No comments yet
Rheology – not to be confused with theology – represents an important and distinctive area of modern engineering science: the ability to specify and control a material’s rheology and associated microstructures is a key aspect of many process and product innovations. In the pharmaceutical sector, emulsions and the cohesive wet masses employed in extrusion processes for granulation – often called pastes – are examples of materials with complex rheological behaviour. Understanding these behaviours, being able to quantify them and then incorporating that knowledge into appropriate models will allow optimisation of steps such as process design, modelling and development, as well as improving product quality, efficiency and time of processing in manufacturing.
Rheology – not to be confused with theology – represents an important and distinctive area of modern engineering science: the ability to specify and control a material's rheology and associated microstructures is a key aspect of many process and product innovations. In the pharmaceutical sector, emulsions and the cohesive wet masses employed in extrusion processes for granulation – often called pastes – are examples of materials with complex rheological behaviour. Understanding these behaviours, being able to quantify them and then incorporating that knowledge into appropriate models will allow optimisation of steps such as process design, modelling and development, as well as improving product quality, efficiency and time of processing in manufacturing.
Rheology – not to be confused with theology – represents an important and distinctive area of modern engineering science: the ability to specify and control a material’s rheology and associated microstructures is a key aspect of many process and product innovations. In the pharmaceutical sector, emulsions and the cohesive wet masses employed in extrusion processes for granulation – often called pastes – are examples of materials with complex rheological behaviour. Understanding these behaviours, being able to quantify them and then incorporating that knowledge into appropriate models will allow optimisation of steps such as process design, modelling and development, as well as improving product quality, efficiency and time of processing in manufacturing.
Several factors are currently prompting pharmaceutical companies to improve their understanding of their manufacturing processes. Production costs are increasing and failing to meet product specification can seriously affect a line’s profitability. The time taken to change from development to manufacture represents time lost on a patent, so scaling up of processes must be reliable with minimal trial and error. There is therefore a need to understand, measure and model the way materials and machines interact, particularly when unfamiliar materials are involved. Furthermore, the drive to develop Process Analytical Technologies (PAT) for intelligent monitoring of pharmaceutical processes requires companies to understand the mechanisms operating in their processes and how these impact products (see Dr Ali Afnan’s article in this issue). An important question is always ‘What to measure?’ and in the case of rheology, the measurement technique depends strongly on the material as experimental data can be interpreted in several ways, depending on one’s assumptions about the nature of the material. Finally, companies can protect or extend their Active Principal Ingredient (API) formulations by clever processing, exploiting the material’s rheology to give innovative products that are less readily duplicated by generic manufacturers. Pharmaceutical pastes are an important example of a group of materials that have been used for some time without a detailed understanding of their rheology, which is now being addressed in earnest.
Pharmaceutical paste processing
A pharmaceutical paste consists of a mixture of API and excipients, both representing the constituents of the dosage form. The process of paste forming involves a preliminary stage in which dry powders including the API are dry mixed by conventional blenders, before addition of a liquid phase and then further wet mixing to ensure homogeneous distribution.1 The solid components in a paste usually feature particle sizes between 0.1 and 100 µm and can either be fully dense or contain internal pores. The particles are usually rigid but in some cases they can deform readily: the proportion of powders and liquid are selected to render the resulting material plastic and soft. (Figure 1)
In the world of rheology, plasticity refers to the ability to withstand a stress without deforming much. This is important in extruded products as they must retain their shape for some time. The complexity arises in linking the properties of the solid and liquid components to the behaviour of the final material, which is a complex function of the solids volume fraction, chemical composition, solubility, particle size distribution and particle shape. The same care must be applied when selecting the liquid phase, as the desired deformation behaviour depends on the type of machine that will process the paste: the near-uniaxial compaction experienced in a tableting machine is very different from the flow and shearing that occurs in, for example, a screw extruder generating extrudate strands for extrusion-spheronisation.
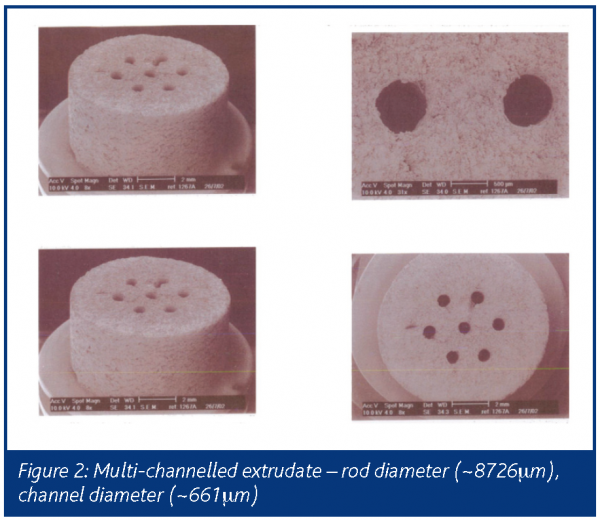
In extrusion a paste is formed into a product of uniform shape, cross-section and density by forcing it through an orifice or die under controlled conditions1. Both the force required for extrusion and the characteristics of the extrudate depend on the rheological properties of the paste, the design of the die and the rate at which the material is pushed through the die. Complex and intricate shapes can be obtained with a high degree of reproducibility, continuously and at high throughput rates. The idea of substituting old batch compression methods with continuous routes for, say, tablets is attracting the attention of process engineers across the pharmaceutical sector. Figure 2 shows a multi-tubular system for controlled release of propranolol hydrochloride, which represents the first example of paste extrusion to yield a solid oral dosage form2. This has potential for continuous production of pharmaceutical tablets: here, the multi-channeled extrudate is followed by finishing steps (drying then coating). The release of the API is mainly controlled by the product geometry. It should be noted that the formulation used for this monolith would not function very well in a normal tableting process as it exhibits very different rheology.
Rheology
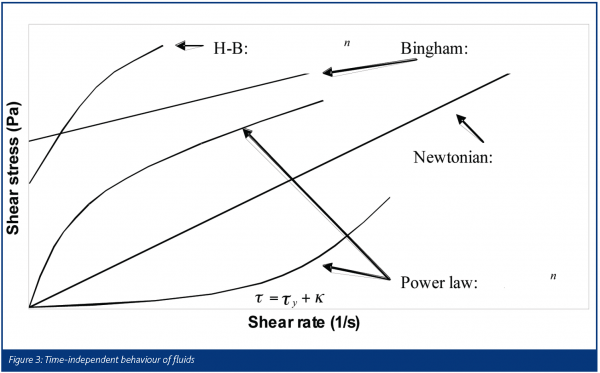
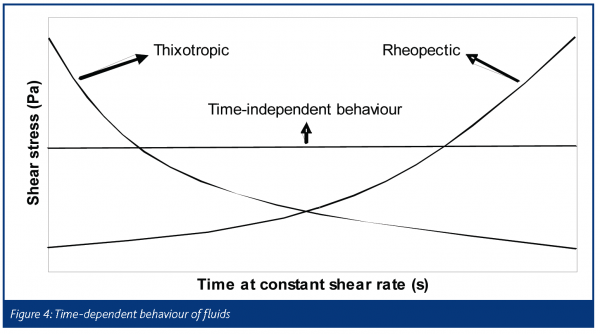
The word rheology comes from the Greek ‘panta rhei’ which means ‘everything flows’. Deformation behaviour is often quantified in terms of the relationship between the stress (τ) of the material and the strain experienced by it (γ). A familiar example is the Hookean law of elasticity, τ = Eγ which is typical for small deformations of solids. Another is Newton’s law of viscosity, τ = μ(γ·), which is observed for many simple liquids. We note an important difference, that solid behaviour is often characterised in terms of strain and fluid behaviour in terms of strain rate. Pharmaceutical pastes, which are highly concentrated suspensions of fine particles, are examples of soft solids, exhibiting features found in both camps. The important parameter is time scale, as some processes such as liquid phase redistribution in the paste, a result of high stresses, are relatively slow whereas elastic compaction (and relaxation) is quite fast. Pastes often behave as homogeneous materials under standard experimental conditions and are often described as single phase visco-plastic fluids. Some attempts have been made to model them as a combination of both phases, e.g. as. Figure 3 shows the more common ‘flow curves’ (shear stress-strain rate plots) observed for time-independent fluids. The Herschel-Bulkley and Bingham models are examples of ‘plastic’ fluids that exhibit a minimum stress level – the yield stress τ = kγsγ1 – which must be overcome in order to deform the material. Figure 4 illustrates some forms of time-dependent behaviour for fluids being sheared at constant strain rate. Unlike polymers, which can exhibit very strong time dependency effects as a result of viscoelasticity, pastes are often weakly time dependent unless the liquid phase is free to move or the system is reactive.
With pharmaceutical pastes, shear-thickening or rheopectic behaviours are to be avoided as a large increase in viscosity would affect both the speed and the integrity of the process due to the high stresses developed. Note also that in extrusion some regions of the material will experience low shear rates and the local strength will therefore vary.
Measurements
In a rheological test, a sample of material is subjected to a controlled strain (or stress) and its stress (or strain) response is measured. The data are then interpreted in terms of constitutive models such as those in Figure 3, which have been used in mathematical simulations of the test in order to relate the geometry and processing conditions to the observed forces and strains. There is always a degree of faith – i.e. theology – involved in interpreting the results. This is particularly true with pharmaceutical pastes, as these materials may not stick to the walls of the test system but slip instead, or the material may not be homogeneous as a result of liquid phase redistribution induced by the test. This can only be confirmed by imaging the material during testing using, for example, magnetic resonance imaging techniques, or by inspection of materials after testing. One must therefore be very careful in selecting an appropriate test and interpreting the data correctly.
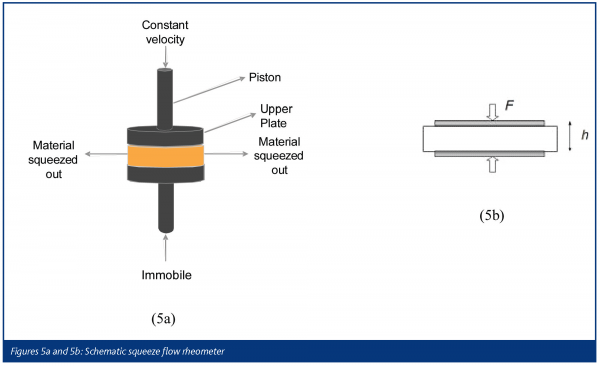
One method for characterising the rheology of materials exhibiting a yield stress, e.g. pharmaceutical pastes, is squeeze flow rheometry (SFR). Figures 5a and 5b show the test geometry, where a fixed cylindrical volume of material is deformed between two flat, coaxial circular plates approaching each other at constant velocity.
As the plates approach, the material is squeezed out with a force (F) inversely proportional to the separation distance (h), which arises due to both bulk shear and extensional deformations. A condition of completely pure shear or purely extensional deformation is never achieved but approximations to one of these models can be reached by using thin specimens and rough plates (shear) or tall specimens and smooth plates (extension). Fortunately, the equations for SFR have been obtained for both wall conditions and shear modes. One must still perform careful experiments!
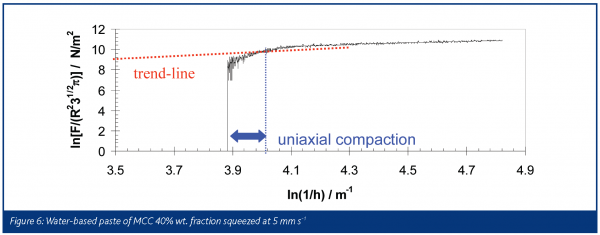
Figure 6 is an example of how a tall sample of water-based microcrystalline cellulose paste, 40% solid weight fraction, can be described using a model based on full slip boundary condition proposed by Chan and Baird for Herschel-Bulkley fluids:
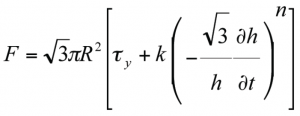
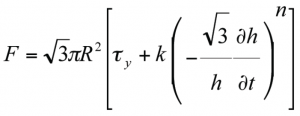
Here, F is the force required to squeeze the paste, R is the radius of the plates and h is the separation of the plates at time t. The experiments were performed using fully lubricated PTFE plates in order to approximate the slip boundary condition. The initial increase in scaled force is not included in the analysis because visual inspection confirmed that the material was not flowing at this point: it was undergoing uniaxial compaction to its shear strength before starting to flow. The figure also shows that a trend-line can be fitted to the remaining data to give the Herschel-Bulkley power index (n = 0.65) and flow consistency (k = 7.5 / f(n) SI units) from the gradient and the intercept respectively.
The value n, which in this case is less than 1, clearly highlights the shear-thinning behaviour of this pharmaceutical paste. Therefore this specific formulation has been found to be consistent with a shear-thinning Hershel-Bulkley model. SFR is an example of how rheological analyses of soft solid materials can be performed well by generating both shear and extensional deformation that are found in extrusion conditions as well.
Formulations
Understanding the deformation and flow behaviour of pharmaceutical pastes is something that must be achieved through a full investigation and evaluation of rheological parameters such as yield stress (τy), wall shear stress (τw), viscosity (η) and so forth. Both bulk and interface rheology must be considered in order to work out the flow boundary conditions and predict the force contributions involved in product shaping. Notwithstanding the fact that process conditions (e.g. process velocity, temperature etc.) and equipment design (e.g. type of extruder) affect the flow behaviour, modifications in formulations hardly influence the rheology as well. For instance, an increase in solid volume fraction of the paste leads to higher values of apparent viscosity (ηa) due to a higher number of inter-particle interactions. This would result in an increase in the magnitude of both τy and τw with a consequent rise in the force required for the design process. The viscosity, in fact, is assumed to be the sum of two components: ho, which is the liquid viscosity and hi, which is the viscosity contribution due to the inter-particle interactions. Paste rheology is also strongly affected by particle size, particle shape and particle size distribution, which each play a key role in the initial compaction of the paste and its dilation during deformation.
In conclusion, it is widely known how complex it is to model the flow behaviour of a paste. Although single phase models have mostly been used so far (i.e. visco-plastic constitutive equations used in fluid mechanics), it would be very challenging to try treating pastes as three phases materials (i.e. containing solid, liquid and air). Each of these phases modifies to a certain extent the rheology of the system.
It is clear that, for pharmaceuticals, in order to shift from batch to continuous production using paste flow mechanisms, an understanding of the paste rheology must be gained.
Acknowledgements
Many thanks to Drs. Sarah Rough, Ian Wilson and Mr. Milan Patel for useful comments.






References
- K. E. Fielden and J. M. Newton. (1992). Extrusion and Extruders. Encyclopedia of Pharmaceutical Technology, vol. 5. 1992, 395-441
- Carter, R.E., Newton, J.M., Caminho, A.M. and Mascia, S. (2004). A novel system for the controlled release of drugs. Pharmaceutical Technology Europe, Dec-2004, 23-29



