Data from four trials support the safety and tolerability of hESC-derived retinal pigment epithelium
Posted: 25 June 2015 |
Results of four trials provide additional evidence supporting the safety and tolerability of hESC-derived retinal pigment epithelium…
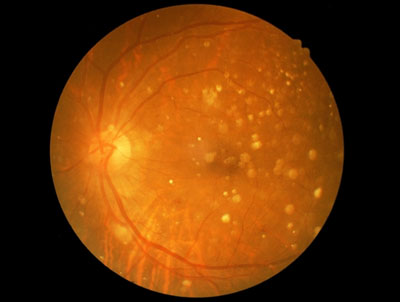

Ocata Therapeutics has announced that data will be presented in a Late Breaking Abstract at the International Society for Stem Cell Research (ISSCR) 13th Annual Meeting being held in Stockholm, Sweden, June 24-27.
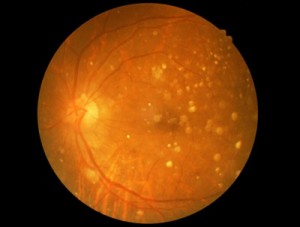

The presentation will provide a follow-up of four prospective safety trials carried out in the United States and also in South Korea. The results of these trials in 31 patients with dry Age-related Macular Degeneration (AMD) and Stargardt’s Macular Dystrophy (SMD) provide additional evidence supporting the safety and tolerability of hESC-derived retinal pigment epithelium (RPE). Some patients were followed for up to 4 years and none of the patients showed evidence of hyperproliferation, rejection or serious adverse ocular or systemic safety issues related to the transplanted tissue.
hESC-derived cells could provide a potentially safe new source of cells for regenerative medicine
The abstract reported that all of the patients, 26 from the US studies and 5 from the South Korean study, experienced improved or stable, best-corrected visual acuity (BCVA). These studies suggest that hESC-derived cells could provide a potentially safe new source of cells for regenerative medicine.
“This late breaking abstract presented at the leading stem cell meeting is indicative of the significance and importance of our new potential breakthrough treatments for these disabling diseases where there is no cure available today,” said Paul K. Wotton, Ph.D., President and Chief Executive Officer. “We now look forward to the planned initiation of our Phase 2 safety study in dry AMD and our pivotal study in SMD.”




