Lapatinib and trastuzumab shrinks HER2+ breast cancers within 11 days
Posted: 11 March 2016 | | No comments yet
A trial has shown that lapatinib plus trastuzumab before surgery shrinks and may even destroy tumours in women with HER2+ breast cancer within 11 days. The EPHOS B trial studied 257 women with HER2 positive breast cancer in the short gap between initial diagnosis and surgery to remove their tumours. The trial set out to study the biological effects of the drug combination by measuring biological markers of cellular proliferation after 11 days of therapy…

A trial has demonstrated that the drug combination of lapatinib and trastuzumab (Herceptin) before surgery shrinks and may even destroy tumours in women with HER2 positive breast cancer within 11 days.


The EPHOS B trial, led by researchers at The Institute of Cancer Research, London, the University of Manchester and University Hospital of South Manchester NHS Foundation Trust, studied 257 women with HER2 positive breast cancer in the short gap between initial diagnosis and surgery to remove their tumours.
In the trial, women were split into three groups and treated for 11 days before their surgery. Initially women were randomised to receive either trastuzamab, or lapatinib or no treatment – but halfway through the trial after evidence from other trials of the effectiveness of the combination the design was altered so that additional women allocated to the lapatinib group were also prescribed trastuzumab.
“Dramatic responses”
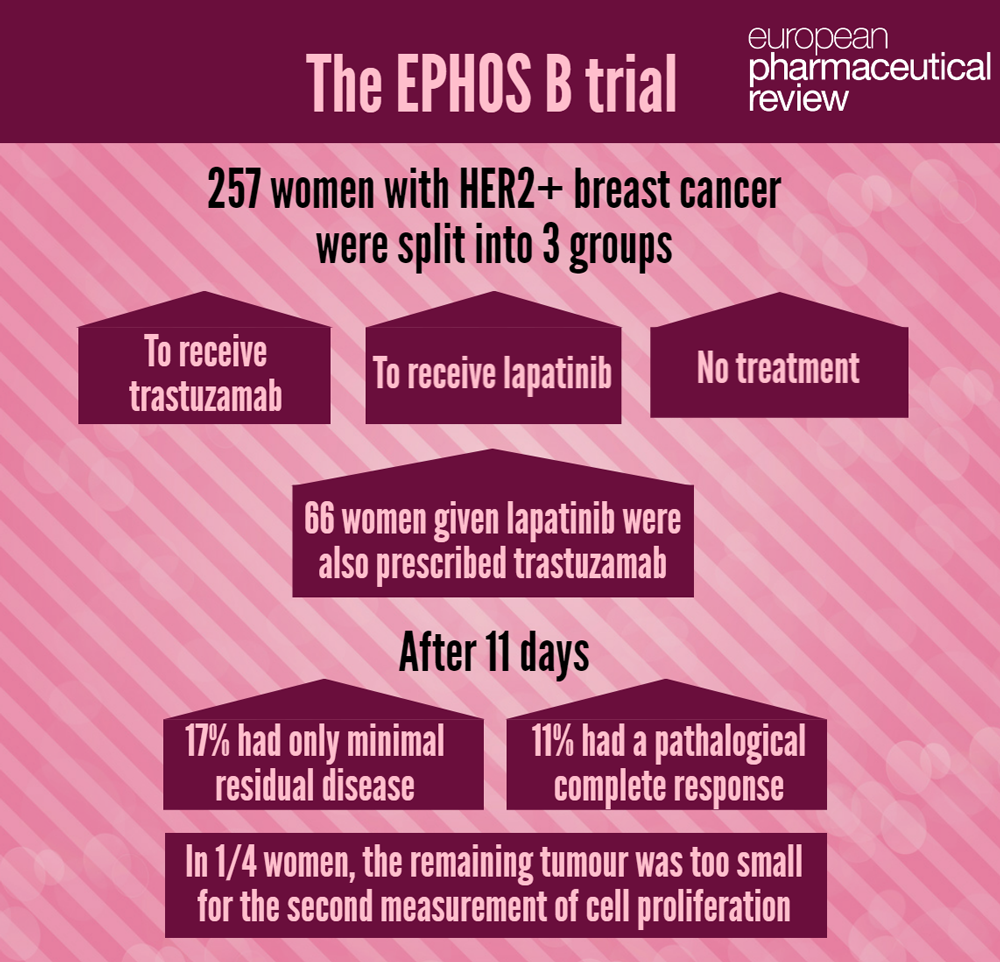

The trial set out to study the biological effects of the drug combination by measuring biological markers of cellular proliferation after 11 days of therapy. But when trying to measure this, the researchers discovered that in roughly a quarter of the 66 women who received both drugs the remaining tumour was too small for the second measurement of cell proliferation.
Seventeen percent of the women receiving both drugs had only minimal residual disease – defined as an invasive tumour smaller than 5mm in size – and 11 percent had a pathological complete response.
Three per cent of the women treated with trastuzumab only had residual disease or complete response.
Commenting on the study, trial co-leader Professor Judith Bliss, director of the Cancer Research UK-funded clinical trials and statistics unit at The Institute of Cancer Research, London, said: “Our trial set out to try to use the window between diagnosis and surgery to find clues that combined treatment with trastuzumab and lapatinib was having a biological effect on HER2 positive tumours. So it was unexpected to see quite such dramatic responses to the trastuzumab and lapatinib within 11 days.
“Our results are a strong foundation on which to build further trials of combination anti-HER2 therapies prior to surgery – which could reduce the number of women who require subsequent chemotherapy, which is also very effective but can lead to long-term side effects.”
Professor Arnie Purushotham, senior clinical adviser at Cancer Research UK, said: “These results are very promising if they stand up in the long run and could be the starting step of finding a new way to treat HER2 positive breast cancers. This could mean some women can avoid chemotherapy after their surgery – sparing them the side-effects and giving them a better quality of life.”