Osteomyelitis: How artificial bone grafts are filling the gap
Posted: 17 November 2016 | | No comments yet
We caught up with Bonesupport’s CEO Richard Davies to find out how they’re using artificial bone grafts to help sufferers of chronic osteomyelitis…

Osteomyelitis is a bone infection, usually caused by bacteria. The most common signs of osteomyelitis are bone pain and a high temperature. There are two ways the infection can occur: following an injury (known as contiguous osteomyelitis) such as a fractured bone, animal bite or during surgery or via the bloodstream (known as haematogenous osteomyelitis).
Once chronic osteomyelitis is established, the person affected may have periods of almost no symptoms. However, symptoms can flare up at any time. For example, you may experience:
- bone pain
- feeling persistently tired
- pus draining from the sinus tract (a passageway that develops near the infected bone)
- local swelling
- skin changes
- excessive sweating
- chills
Source: NHS
We caught up with Bonesupport’s CEO Richard Davies to find out more about the infection, what’s in the pipeline for Bonesupport and how they are already are helping to fill in the gaps and heal patients…
What are the main aims of Bonesupport?
Bonesupport is a fast growing orthobiologics company. We are well positioned to continue our rapid growth based on the competitive advantages that our current line-up of Cerament products deliver both in terms of clinical outcomes and value.
We are focused on ensuring that more patients have access to our products given the clear clinical benefits that they deliver in the filling of bone voids caused by major orthopaedic indications such as trauma, revision arthroplasty and chronic osteomyelitis.
ARVE Error: url: https://www.youtube.com/feeds/videos.xml?channel_id=UCXv6DOw Status code 200 expected but was 404.What are your products used for?
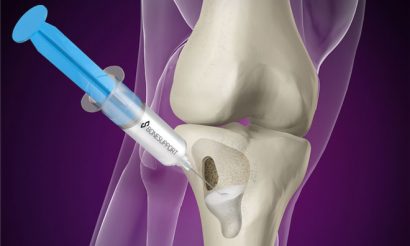

Injecting Cerament G into the bone void
Cerament is an osteoconductive biomaterial with a biphasic action allowing for controlled resorption of the material with parallel bone ingrowth and remodelling.
Our products are used by orthopaedic surgeons’ in their everyday management of 2 major conditions:
- Filling of bone voids. Cerament BVF is able to fill the void completely and then remodels to host bone in 6 to 12 months
- Managing or preventing bone infections associated with the management of bone voids – bactericidal, sustained local bone antibiotic levels above the MICs of potential pathogens
Who benefits medically from your products? Describe the typical patient.
Our products are used to manage bone voids associated with trauma, patients undergoing revision arthroplasty and patients suffering from osteomyelitis or infected diabetic foot ulcers.
One indication where the Cerament G has recently been shown to be of great value is in the management of chronic osteomyelitis. A paper published by Mr Martin McNally, Consultant Bone Infection and Limb Reconstruction Surgeon at Oxford University Hospitals (Oxford, UK) in The Bone and Joint Journal. (McNally et al, The Bone and Joint Journal, 2016, Vol. 98-B, No. 9, p1289-96)
This paper provided 12-34 month follow up data from the first 100 patients in a prospective study evaluating Cerament G for dead space (void) management in patients with chronic osteomyelitis using a single stage surgical procedure.
The mean duration of chronic osteomyelitis in this patient group was 10.4 years (0.5 to 68 years). These data showed that this surgical approach, augmented by the use of Cerament G, was highly effective, delivering a 96% prevention of infection recurrence rate, a 3.0% fracture rate and a total wound leakage rate of 6.0%. This is significantly lower than published results with alternative bone graft substitutes that deliver antibiotics locally.
CASE STUDY
Patient: 64 year old male
Diagnosis:
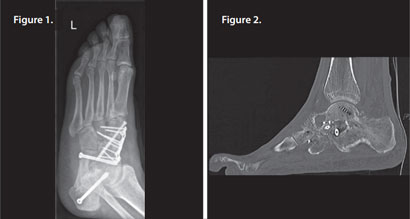

Patient had pes planus (flat foot), treated by arthrodesis of the talonavicular joint and subtalar joint. Some redness and swelling was initially noted but this settled without therapeutic intervention.
Three months later, infection with Staph aureus was confirmed and a CT scan showed arthrodesis, but with significant osteolysis present around the metalwork (Figures 1 & 2).
Treatment:

All metalwork was removed, a thorough débridement was carried out and three Septopal chains (containing gentamicin) were inserted in the first part of a two-stage procedure. The patient was given systematic cefazolin.
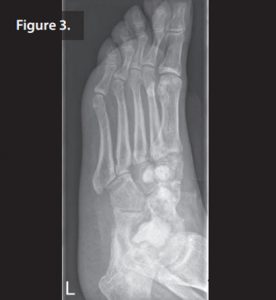
During outpatient follow up the patient had clear wound secretion and primary healing of the wound with negative pressure wound therapy. 5 weeks post-operatively, the wound had completely healed and the patient had normalised inflammatory markers. R During the second stage, the Septopal chains were removed, another débridement was carried out and the remaining defects filled with 10mL of Cerament G (Figure 3).
Outcome:

The patient had white wound drainage post-operatively but the wound healed with negative pressure wound therapy, and the patient was allowed to partially weight bear using a walker.
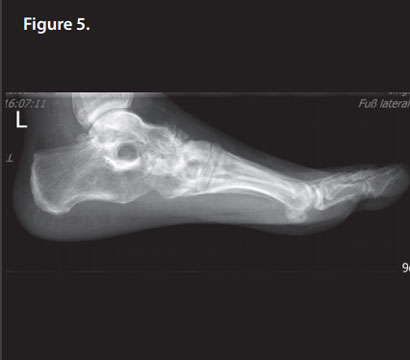
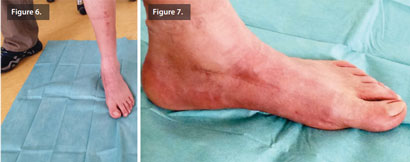
After 6 weeks, the patient was fully weight bearing. R 5 months post-operatively, bone remodeling can be seen on the X-rays where Cerament G was injected (Figure 5), and the patient is pain and infection-free, with a complete return to normal activity and footwear (Figures 6 & 7).

Images and case study sourced from Bonesupport
Why are calcium sulphate and hydroxyapatite the main components of Cerament?
Cerament consists of 60% calcium sulfate and 40% hydroxyapatite.
Both components play an important role in how our products are able to remodel to host bone within 6 to 12 months.
The calcium sulfate:
- Delivers hydroxyl apatite into the bone void that is being treated
- Leads to controlled host bone ingrowth as it is gradually resorbed
- Provides initial stability
The hydroxyapatite forms a scaffold to which osteoblasts attach to form new host bone.
What do you hope to achieve in the next five years with Bonesupport?
We have a very clear strategy for the company which is based around:
- Driving sales growth of our existing products
- Improving the clinical and value proposition that our products provide by generating more supportive clinical data in trauma, revision arthroplasty and infected diabetic foot ulcers
- Gaining US approval for Cerament G based on the successful completion of the FORTIFY PMA study which is due to start before the end of 2016
- Building a pipeline of new product opportunities based on the drug eluting properties of our Cerament platform.
We have recently raised $37 million which will allow us to fund the execution of this strategy.
And finally, what is your ultimate goal for your career?
I am focused on building Bonesupport’s business. I believe very strongly that our products can deliver major improvements to the lives of orthopaedic patients given their ability remodel to host bone and to prevent or manage highly debilitating bone infections, including chronic osteomyelitis.
We also have the potential to help even more patients as we look to leverage the drug -eluting properties of the Cerament platform to extend our product offering.
With such an exciting future I am committed to growing Bonesupport into a global leading orthobiologics company.


Richard Davies joined Bonesupport as Chief Executive Officer in January 2016. As a senior commercial leader in the life sciences industry, he brings 25 years of global experience in all aspects of sales / marketing and country / regional leadership, with a track record of devising and executing growth strategies and delivering successful results in complex situations.