Raman spectroscopy in pharmaceutical analysis
Posted: 9 October 2009 | Babur Z. Chowdhry, Professor of Biochemistry, University of Greenwich; Saima Jabeen (PhD), Research Assistant, University of Greenwich and Bruce Alexander (PhD), Lecturer in Physical Chemistry, University of Greenwich | No comments yet
A wide and versatile range of analytical techniques are routinely used, indeed are necessary, in pharmaceutical analysis. Over the past decade Raman spectroscopy has increasingly come to the fore as a valuable member of the arsenal of methods used, from both a fundamental and applied perspective, for the interrogation of solid, liquid and solution phase samples. Advances have occurred not only in instrumentation but also in fundamental techniques and applications. The method holds substantial potential for the investigation of, what are normally considered, problematic or challenging areas of analysis. The aforementioned areas include – but are, definitely not limited too reaction kinetics, pharmaceutical drug discovery, detection of counterfeit/adulterated/illegal drugs, trace analysis and uses for on-line pharmaceutical process manufacturing. This, the first of several articles on the use of Raman spectroscopic techniques in pharmaceutical analysis, provides an introductory overview of the theory of the technique.
A wide and versatile range of analytical techniques are routinely used, indeed are necessary, in pharmaceutical analysis. Over the past decade Raman spectroscopy has increasingly come to the fore as a valuable member of the arsenal of methods used, from both a fundamental and applied perspective, for the interrogation of solid, liquid and solution phase samples. Advances have occurred not only in instrumentation but also in fundamental techniques and applications. The method holds substantial potential for the investigation of, what are normally considered, problematic or challenging areas of analysis. The aforementioned areas include - but are, definitely not limited too reaction kinetics, pharmaceutical drug discovery, detection of counterfeit/adulterated/illegal drugs, trace analysis and uses for on-line pharmaceutical process manufacturing. This, the first of several articles on the use of Raman spectroscopic techniques in pharmaceutical analysis, provides an introductory overview of the theory of the technique.
A wide and versatile range of analytical techniques are routinely used, indeed are necessary, in pharmaceutical analysis. Over the past decade Raman spectroscopy has increasingly come to the fore as a valuable member of the arsenal of methods used, from both a fundamental and applied perspective, for the interrogation of solid, liquid and solution phase samples. Advances have occurred not only in instrumentation but also in fundamental techniques and applications. The method holds substantial potential for the investigation of, what are normally considered, problematic or challenging areas of analysis. The aforementioned areas include – but are, definitely not limited too reaction kinetics, pharmaceutical drug discovery, detection of counterfeit/adulterated/illegal drugs, trace analysis and uses for on-line pharmaceutical process manufacturing. This, the first of several articles on the use of Raman spectroscopic techniques in pharmaceutical analysis, provides an introductory overview of the theory of the technique.
The Raman effect
When electromagnetic radiation of UV, visible or near infrared wavelengths interacts with matter, the photons involved are either absorbed or scattered. Of those which are scattered, the vast majority of photons are scattered elastically (i.e. have the same energy (frequency) and, therefore, wavelength, as the incident photons). Depending on a number of factors, (principally the size of the scattering particle and its refractive index relative to that of the surrounding medium), different scattering processes can occur; the most common for molecular entities being Rayleigh scattering, where the scattered light has the same energy as the incident light. However, a small fraction of photons (approximately one in 105-107 photons) are scattered inelastically giving rise to the Raman effect. Clearly this is a small fraction of electromagnetic radiation that is scattered with a different energy to the Rayleigh scattering – the Raman cross-section is ≈10-32-10-29 cm, the Raman signal is, therefore, intrinsically weak. In comparison, fluorescence (which can be a competing process) has a much greater cross-section (≈10-17 cm) and therefore, when present, can overwhelm the Raman signal. For Raman, the scattering event occurs in ≈10-14 seconds or less (cf, fluorescence: 10-10-10-7 seconds). Raman scattering can occur with a change in vibrational, rotational or electronic energy of a molecule. Herein we are concerned primarily with the vibrational Raman effect. The difference in energy between the incident photon and the Raman scattered photon is equal to the energy of a vibration of the scattering molecule. A plot of signal intensity of scattered light versus wavenumber or Raman shift results in a Raman spectrum.
Off-resonance (i.e. or “normal”/”back-scattered”) Raman scattering can be described by the following process. Upon absorption of light, an electron is excited from the ground state to a virtual state. This virtual state typically lies between the ground state and the first excited electronic state. Upon relaxation from the virtual state, a photon is emitted. This emission can be classified as Stokes scattering, which is red-shifted in relation to the incident light, or anti-Stokes scattering, which is blue-shifted in relation to the incident light. Experimentally, the former is measured almost exclusively because, at room temperature, the Boltzmann distribution dictates that very few molecules will be in the upper levels of the ground state compared to the lower levels of the ground state (see Figures 1 and 2) as such, the intensities of anti-Stokes scattering are normally quite weak compared to Stokes scattering.
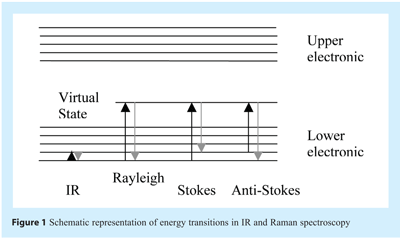

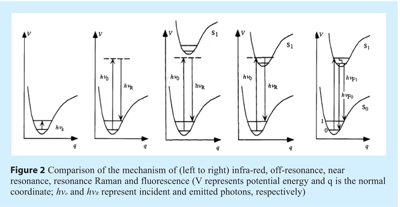

When a molecule is exposed to an electric field, electrons and nuclei are forced to move in opposite directions. A dipole moment is induced which is proportional to the electric field strength and to the molecular polarisability α. The dipole moment, P, induced in a molecule by an external electric field, E, is proportional to the field as shown in the following equation:
P = αE
The proportionality constant α is the polarisability of the molecule; at a “simple” level it is a measure of the ease with which an electron cloud around a molecule can be distorted. Raman scattering occurs, therefore, because a molecular vibration can change the polarisability ellipsoid, which expands and contracts when the bond length between two atoms increase or decreases, respectively, due to a vibration. Because α does not vary significantly as a function of inter-nuclear distance (at least, not to the same extent as the dipole moment in infra-red spectroscopy) vibrational Raman intensities are less sensitive to the environment, e.g. solvent medium, compared to infra-red spectroscopy.
Near resonance and Resonance-Enhanced Raman Scattering
If the wavelength of the exciting laser is close to, or within, the allowed electronic spectrum of a molecule, near resonance or resonance enhancement (i.e. resonance) Raman occurs, respectively (see Figure 2). In the latter case, the intensity of some Raman-active vibrations can increase by a factor of ≈103-105. Despite the fact that many molecules absorb in the ultraviolet (such as peptides), the high cost of lasers and optics for this spectral region has limited UV resonance Raman spectroscopy to a small number of specialists.
Polarisation effects
Raman scatter is partially polarised, even for molecules in a liquid where the individual molecules are randomly oriented. The effect is most easily seen with an excitation source which is plane-polarised. In isotropic media, polarisation arises because the induced electric dipole has components which vary spatially with respect to the coordinates of the molecule. Raman scatter from totally symmetric vibrations will be strongly polarised parallel to the plane of polarisation of the incident light. This strong polarisation dependence of the intensity of these modes has the effect of assisting with identifying the molecular vibrations responsible for the Raman bands. The scattered intensity from non-totally symmetric vibrations is three quarters as strong in the plane perpendicular to the plane of polarisation of the incident light as in the plane parallel to it. In a crystalline material the orientation the crystal is fixed in the optical system and the arrangement of molecules in the laser beam is no longer random. The polarisation components depend on the orientation of the crystal axes with respect to the plane of polarisation of the input light, as well as on the relative polarisation of the input and the observing polariser. This has important, and unfortunately often overlooked, consequences when considering polymorphic materials. For example, when studying the distribution of a crystalline material in a solid matrix, intensity fluctuation can arise due to the random orientation of crystallites in a sample, rather than any inherent chemical differences between the crystallites.
Surface-Enhanced Raman Scattering
Another method for enhancing the normally weak Raman signal is surface-enhanced Raman spectrosopy (SERS). This technique involves the adsorbtion of molecules on certain rough metallic substrates. As a result of large optical fields and resonance related effects induced by the coupling of the incident laser light to the metal surface, the Raman cross-section for a molecule on a substrate surface may be enhanced by factors of up to 106-108 (or more). Since the discovery of SERS the exact cause of the enhancement mechanism is still under debate due to the fact that there is undoubtedly more than one effect responsible for signal enhancement. Nevertheless, it has been established that SERS occurs though a combination of both a long-range electromagnetic effect (EM) and a short-range chemical effect (CT).
Generally, it is accepted that the major signal enhancement contribution observed in SERS results from the EM effect. The EM effect occurs when the laser radiation is in resonance with a surface plasmon excitation of the metal. A surface plasmon is a collective excitation of the free electrons in a metal and the frequency at which this occurs is usually much lower at the surface than in the bulk, and often corresponds to light in the visible region of the electromagnetic spectrum. Essentially, the EM effect arises from localisation of the surface plasmon resonance which effectively traps photons and increases the probability of a Raman interaction with vibrational excitations of neighbouring molecules. Because the nature of the substrate material, as well as its size, shape, and dielectric environment dictate the characteristics of the localised surface plasmon resonance; these properties also control the surface-enhancing capability of the SERS substrates.
The exact origin of the chemical or electronic mechanism is still open to some debate. The predominant view suggests that the enhancement is due to the formation of a charge transfer (CT) complex between atomic scale roughness features on the metal surface (adatoms) and the adsorbed molecule. The energies of the frontier molecular orbitals of the adsorbed molecule are close to the Fermi level of the metal because of complex formation. The difference in energy between the Fermi level and the frontier orbital of the adsorbed species is close in frequency to the incident light, and an enhancement mechanism occurs, which is analogous to the resonance Raman process. The most effective SERS substrates have been found to be copper (Cu), silver (Ag) and gold (Au) (i.e. coinage metals/noble metals), which all exhibit surface plasmon resonance in the visible region.
Surface enhanced resonance Raman scattering, SERRS, occurs when the SERS effect is coupled with resonance Raman, i.e. when an electronic transition of an adsorbed molecule is excited by the incident beam. This leads to a further increase in the signal intensity as the two enhancement mechanism can be considered cooperative.
The total SER(R)S enhancement (compared to dispersive solution state Raman) depends on a number of factors, the most important include: nature of analyte and the adsorption mechanism (i.e. physi-adsorbed or chemi-sorbed); chemical, optical, and morphological properties of the substrate; environmental solution conditions, and the nature of the exciting radiation. Molecules with lone pair electrons or π clouds show the strongest SERS as they tend to be favourably bound to metal surfaces, and as such are in close proximity to the substrate, e.g. aromatic nitrogen or oxygen containing compounds (such as aromatic amines or phenols) are strongly SERS active. The effect can also been seen with other electron-donor functionalities such as carboxylic acids or thiols.
Due, as stated above, to the fact that Raman is a weak effect its general use amongst the academic and applied scientific community did not start to occur until the late sixties and early seventies; unlike e.g. infra-red spectroscopy which was by that time well established. One of the primary reasons for this being, despite the fact that the technique was first reported from an experimental viewpoint in 1928 by C.V. Raman, suitable commercially available instrumentation was difficult to obtain and the technique was virtually unknown except for specialised users. Since the early seventies, however, there has been a rapid growth in the use of Raman in virtually all areas of the scientific community relating to the physical and chemical sciences, including pharmaceutical science. This development occurred due to many factors, primary amongst them being the availability of lasers and Raman detection equipment, such as photomultipliers and CCD detectors. The use of lasers in contemporary Raman spectroscopy, as a source of incident photons, is now absolute. Briefly, the properties that distinguish lasers from ordinary light sources are that they are brighter, tunable and (perhaps most importantly) highly monochromatic and directional. They also allow polarisation control and can probe molecules on the femtosecond (10-15 s) time-scale. Lasers that are commonly used in Raman spectroscopy span the range from the UV to the near infra-red, and most spectrometers are fitted with lasers that emit visible light. However, many compounds fluoresce when illuminated by visible lasers. This problem can be overcome, or minimised, via the use of a near infra-red laser to illuminate the sample in order to obtain Raman spectra.
Spectral information
The information contained in a Raman spectrum is predominantly that concerning molecular vibrations, even although the Raman process is one which is principally an electronic transition. The motions of the atoms in a molecule relative to other nuclei in the same molecule can be considered to be a superposition of normal vibrations. Non-linear and linear polyatomic molecules consisting of N atoms possess 3N-6 or 3N-5 normal vibrations, respectively. The Raman shift, measured in wavenumbers, of bands in a Raman spectrum are dependent on the masses of the atoms involved in the vibrational motion and the elastic forces between them i.e. force constants. For a diatomic molecule the wavenumber or frequency can be calculated as follows:
![]()
![]()
Where ν~ is the wavenumber, n the frequency, and c speed of light (i.e. 3×108 m s-1), k is the force constant and μ the reduced mass of the molecule (equal to m1m2/(m1+ m2) where m1 and m2 are the masses of the atoms.
Many different vibrational modes occur simultaneously for any molecular system, and many are localised on specific functional groups. As such they can give Raman bands that occur at energies that are characteristic of that functional group. Examples are shown in Figure 3.
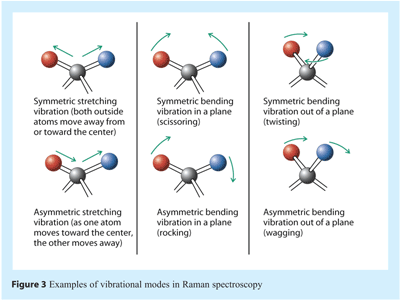

The energy of a vibrational mode, i.e. the motion of the nuclei, depends on molecular structure and environment. Atomic mass, bond order, molecular substituents, molecular geometry and hydrogen bonding all affect the vibrational force constant which, in turn dictates the vibrational energy. The wavenumber of a vibrational band, i.e. the signal that appears in a spectrum, gives the energy associated with a particular excitation, whereas the intensity reflects the “ease of transition” and the concentration of the vibrational band excitation. Bond stretching requires shorter wavelength radiation (higher frequency and higher energy) than bond bending. For example C≡C absorbs at a higher frequency than C=C, which in turn is higher than C-C. Furthermore, the smaller the reduced mass of a system the greater the energy required to cause vibrational excitation and the higher the frequency of radiation required necessary. O-H and C-H stretching occurs at higher frequency than C-O>C-C. O-H stretching should occur at higher frequency than O-D. The vibrations of a highly polar moiety, such as the O-H bond, are usually weak in Raman spectra. Every molecule has slightly different vibrational modes compared to any other molecule. Thus, the Raman spectrum of a given molecule, collected under specified experimental conditions and instrumentation, is often referred to as being unique.
Both infrared and Raman spectroscopies contain information regarding molecular vibrations. As such, they can afford “similar” data. However, the two techniques are complimentary as it is often the case that different vibrational modes are probed by the two techniques. For example, if a molecule has a centre of symmetry vibrations which are Raman-active will be silent in the infrared and vice versa.
Examples of Raman spectra, that of rhodanine and its derivatives, are shown in Figure 4. Rhodanine-based molecules are biologically active and inhibit numerous cellular targets such as HCV NS3 protease, β-lactamase, PMT1 mannosyl transferase, and PRL-3 and JSP-1 phosphatases. The example serves to show not only how Raman spectra differ between molecules which are, structurally, closely related, but also the rich spectral line content.
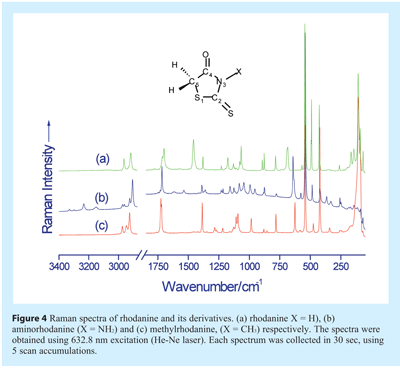

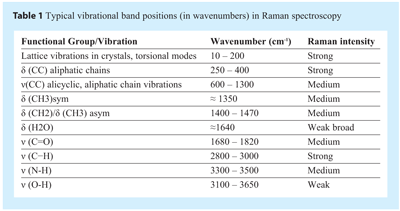
Vibrational bands in Raman spectra often consist of more than one vibrational motion, e.g. some peptides display bands that are due to a mix of N-H bending and C-N stretching motions of the amide group. As such, band assignments may sometimes be difficult even for so called small molecular weight “pure” molecules. Nevertheless, some general comments can be made, regarding the relationship between vibrational bands and wavenumber (see Table 1). In the wavenumber region above 2700 cm-1 the vibrational bands are due to N-H, and C-H stretching modes. The 1500-1700 cm-1 wavenumber region vibrational bands include the following normal modes: C=O and C=N stretch. The C-H bending modes (such as symmetric or asymmetric CH3 and/or CH2 bending vibrations), CH2 wagging and torsion bands typically occur between 1000-1550 cm-1. In the ≈1310-1280 cm-1 region it is common to find CH2 wagging vibrations and in the 900-1200 cm-1 wavenumber region C-C stretching, CH3 and CH2 rocking modes and also C=O out of plane bending can be observed. The 500-900 cm-1 wavenumber region includes C=O wagging and bending modes. Other vibrations in this region include ring bending, deformations, and stretching modes. In the 400 to 500 cm-1 region of the spectra there are quite a number of bands, which can have C-O, C-N, and C-C bending contributions. Ring torsion and lattice vibrational modes are usually found at wavenumbers below 180 cm-1.

Advantages of Raman spectroscopy
There are many advantages of Raman spectroscopy, the most notable being:
- Small amounts (e.g. micromoles) of solid-state samples can be examined, e.g. a single crystal or a pin-head amount of sample.
- 2-dimensional maps can be constructed from a series of Raman spectra taken across a sample surface.
- The technique is non-destructive of the sample.
- No (or minimal) sample preparation is required. Nujol or KBr matrices are not necessary as is the case for conventional IR spectroscopy; the laser is simply directed onto the sample.
- Wet samples or even aqueous solutions, such as typical biological media, can be analysed because water is a particularly poor Raman scatterer.
- In certain cases, in situ analysis is possible with no sample preparation required. Raman spectroscopy can be used to analyse samples through glass and plastic.
- Raman spectrometers fitted with fibre optic probes can be used for remote or on-line analysis.
- Raman bands are narrower than those typically observed in mid-IR spectra and can be used more readily for quantitative analysis.
- The technique is suitable for use with both organic and inorganic materials.
- Raman spectroscopy can measure vibrations from symmetrical molecular modes, which are frequently very weak in IR spectra.
- Raman bands can usually be related more easily, than IR bands, to chemical structure due to the fact that fundamental modes are measured.
- Homonuclear and heteronuclear diatomic molecules give Raman spectra.
Limitations of Raman spectroscopy
Although, Raman is a versatile analytical technique, it suffers from the following disadvantages:
- The Raman effect is a weak effect, which leads to the need for intense laser excitation sources and sensitive detectors.
- Low signal strength, compared with fluorescence. Therefore, conventional Raman is not well suited to identify minor components of a mixture, which is a frequent requirement. FT-Raman has to some extent overcome this problem for “solid” state (powder) samples.
- If laser excitation intensities are too high, they may thermally decompose the sample.
- High concentrations of analytes (solutes) required for solution Raman studies. This problem can be overcome by using resonance Raman or surface enhanced (resonance) Raman spectroscopy (SERS/SERRS).
Future articles in this series will examine Raman instrumentation/band assignments and applications in pharmaceutical science.
Suggested further reading
- Smith E, Dent G. Modern Spectroscopy-A Practical Approach, John Wiley & Sons, (UK) ISBN 10 0-471-49794-0, 2005.
- Colthup NB, Daly LH. Wiberley SE. Introduction to Infrared and Raman Spectroscopy. Academic Press (California, USA) ISBN 0-12-182554-X, 1990.
- Hollas JM. Modern Spectroscopy. J. Wiley & Sons (UK) ISBN 13:978-0-470-84416-8, 2004.
- Ferraro JR, Nakamoto K. Introductory Raman Spectroscopy. Academic Press (California, USA) ISBN 0-12-253990-7, 1994.
- Lambert JB, Shurvell HF, Lightner DA, Cooks GR. Organic Structural Spectroscopy. Prentice Hall (New Jersey, USA) ISBN 0-13-258690-8, 1998.






