Avoiding environmental monitoring ‘false negatives’: overcoming disinfectant residues with culture media neutralisers
Posted: 6 September 2018 | Tim Sandle | No comments yet
Even with the rise of rapid microbiological methods, most environmental monitoring applications are undertaken using culture media, with many alternative methods also being growth-based. This makes the selection, control and release of culture media an area of great importance, given that the quality of the culture media underpins the environmental monitoring programme.


In addition to selecting the right culture media, the use of an appropriate neutraliser is important in relation to surface, and some personnel, monitoring. Neutralisers are required to overcome any residues left by disinfectants, as can be found on cleanroom surfaces or on the gloved hands of personnel.2 The use of a neutraliser within the culture media formulation is also necessary to overcome residues from antimicrobial compounds so that a false negative is avoided. The use of a neutraliser is recommended in the biocontamination control standard ISO 14698;3 and, outside of pharmaceuticals, the cosmetics microbiological test standard ISO 21149 contains some useful advice on neutraliser selection.4
The selection of an appropriate neutraliser is not straightforward. The neutraliser must be non-toxic to the microorganisms expected to be recovered; be able to stop residual disinfectant activity; and, importantly, be effective against each disinfectant in use. This latter requirement often proves the most challenging.
This article examines the most common neutralisers used; some of the problems associated with their selection; and the complexities around using the most appropriate neutralisers in the culture media most commonly used in the environmental monitoring programme.
Culture media and neutralisers
Culture media is required for the cultivation of microorganisms. Media contains the substances that are needed to support growth. Some media are general; others are designed for specific isolation.5 There are a myriad of different types, which reflects the diversity of microorganisms and their different metabolic pathways. With environmental monitoring, there are differences in practice: the use of one universal medium or two (one for the recovery of bacteria and one for the recovery of fungi); differences in incubation temperatures (one or two temperatures, and with the latter the order of incubation); and with incubation time.6 Whichever strategy is adopted, it should be qualified, and the reliability of the culture media must be assured by assessing the culture media supplier through activities such as audits; defined storage conditions; and on-delivery growth promotion testing.
A neutraliser is needed to overcome the inhibitory effect of any residual antimicrobial substance, as might occur with an established test, such as the sterility test, antimicrobial effectiveness test or disinfectant efficacy tests. In the past the term ‘inactivator’ was sometimes used, although ‘neutraliser’ is more common today. Neutralisers are also added to some culture media in case of disinfectant residues when the media is used for the environmental monitoring of surfaces or from the gloved hands of personnel. Neutralisers are also used in pharmaceutical microbiology by being added to rinse solutions, to overcome any residuals that might affix to a membrane filter, and with disinfectant efficacy testing.7
Why neutralisers are required
When sampling the gloved hands of personnel and surfaces using contact plates, it is important that the culture medium contains a suitable neutraliser. This is to address residues of disinfectants that are most likely to be present.8 Most disinfectants will leave some non-volatile residues on a surface after drying. The amount of residue remaining varies depending on the active and product formulation. The type of disinfectant less likely to leave a residue is hydrogen peroxide, which breaks down into water and oxygen relatively rapidly (within 30 minutes on a surface). With other types of disinfectant, residues are likely to remain for prolonged periods. Although some facilities follow disinfectant application with rinsing (either following each application or when changing over between disinfectants), the process is variable and difficult to qualify. It is therefore prudent to consider that any cleanroom surface could contain traces of a disinfectant (Figure 1).


Figure 1: Taking finger dabs in a laboratory
When contact plates are applied to the surface, or larger plates (typically those of a 9cm diameter size) are used to sample gloved hands, the residues will be transferred to the agar and re-solubilised; hence they may inhibit recovery of organisms. The use of contact plates or larger plates for finger dab sampling, containing appropriate neutralisers, can prevent this phenomenon.
The presence of a neutraliser is also required for swabbing. Swabs are commonly either plated out or placed into a medium and subjected to a procedure designed to remove bound organisms from the swab. The medium is then filtered. If swabs are plated the culture medium should contain a neutraliser and if swabs are filtered, the rinse medium should contain a neutraliser. Neutralising additives like polysorbate or lecithin are used to neutralise inhibitory disinfectant residues transferred to the swab during sampling that might inhibit microbial growth.
For the monitoring of process environments where antibiotics are manufactured, the culture medium needs to contain beta-lactamase to neutralise the particular beta-lactam antibiotic being produced, such as penicillins and cephalosporins.9
When neutralisers are not required
Neutralisers are not required in all of the culture media article used. Where there is no need to neutralise, the presence of a neutraliser can simply add unnecessary cost to the culture media. For example, if the same size of plate is used for both surface monitoring and active air-sampling, there would be no need for the active air-sample media to contain a neutraliser. In other cases, the presence of a neutraliser can lead to poor performance from an article. With settle plate exposure, an important consideration is demonstrating that the plate can be exposed for four hours, under unidirectional airflow, without the loss of moisture that occurs leading to the plate being unable to recover any microorganisms that deposit into the plate through microbial carrying particles landing on the plate through gravity. The exposure time, therefore, requires qualifying.10 The presence of a neutraliser in the agar can, in this author’s experience, lead to the agar matrix breaking and, thus, to the plate cracking during post-exposure incubation. An example is shown in Figure 2.
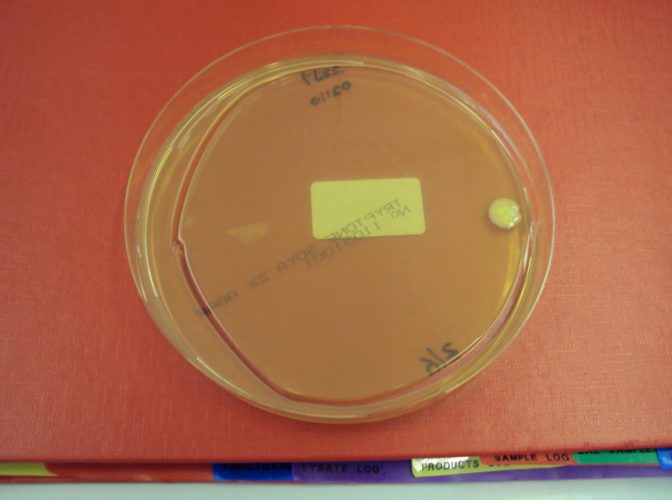

Figure 2: Agar plate used as a settle plate showing signs of cracking, post-incubation (Image: Tim Sandle)
A plate that ‘cracks’ is invalid, and this creates a data integrity issue in relation to the reading of environmental monitoring samples. It is better practice to ensure that agar plates used as settle plates do not contain neutralisers.
Types of neutralisers
While the major pharmacopeia provide some guidance as to neutralisers that can inactivate different antimicrobial substances, these tend to be forms of guidance orientated towards preservatives. Care should therefore be taken with the selection. A complexity with neutralisers is that one neutraliser may be effective against a given disinfectant but ineffective against another. This creates a problem where two disinfectants are used in rotation (and rotation of two different disinfectants with different modes of activity is a regulatory requirement). To avoid the need to use two different contact plates, each containing a different neutraliser, effort should be made to find a universal neutraliser.11 A universal neutraliser is a combination of chemicals, such as lecithin, to neutralise quaternary ammonium compounds, amphoteric surfactants, benzamidines, chlorhexidines and dequadin; and polysorbate 80 to neutralise alcohols and phenolic-based disinfectants.
This selection is not always straightforward and must involve a literature review. The greater challenges arise from disinfectants classed as sporicides. Here, more complex combinations are often required, such as lecithin, polysorbate 80, sodium thiosulphate and L-histidine, which provides the ability to neutralise residues of chlorine-related spoiridical substances in relation to non-sporicidal disinfectants, such as quaternary ammonium compounds, phenolics (of a low pH value), and iodine.12 The presence of sodium thisulphate inactivates sodium hypochlorite and acidified sodium chlorite. This neutraliser is a variant of D / E Neutralising Agar, which was developed by Dey-Engley.13 This formulation is capable of neutralising a broad spectrum of antiseptic and disinfectant chemicals, which extends to the alcohol residues that may be present on the gloved hands of operators in relation to finger plate monitoring. However, for hydrogen peroxide, plates containing a separate neutraliser may be required, such as sodium pyruvate, if the breakdown effect cannot be demonstrated.14 Alternatively, the use of cysteine has been shown to be an effective reducing agent for neutralising oxidising agents such as hydrogen peroxide and iodine.
When the culture media company develops an appropriate neutraliser, the neutralising agent should be added before sterilisation of the media. As part of the development, the neutraliser product should be tested to demonstrate efficacy and absence of toxicity for microorganisms.
Qualifying neutralisers
The culture media supplier should have on file studies that show the suitability of neutralisers in relation to microbial toxicity, using methods such as the quantitative microtitre method or the paper disc assay method. In addition, the facility microbiologist should perform testing to prove that low numbers of organisms can be recovered in the presence of disinfectant residues. Facility isolates should be included in the test panel.
Summary
This article has provided a short overview of neutralisers required for culture media for use with an environmental monitoring programme. The most important points are firstly the need to include a neutraliser and, secondly, selecting the correct type of neutraliser: get this wrong and the validity of tests becomes open to question. Therefore, the experimental design used to establish the efficacy of biocide neutralisation has a major impact on the estimation of antimicrobial efficacy. Microbiologists should work closely with culture media suppliers to ensure that the correct neutralisers are being used.
References
- Sandle T. (2012) ‘Environmental Monitoring: a practical approach’ In Moldenhauer, J. Environmental Monitoring: a comprehensive handbook, Volume 6, PDA/DHI: River Grove, USA, pp29-54.
- Schiemann DA, Chatfield DE. (1976) Evaluation of neutralizers in Rodac media for microbial recovery from disinfected floors, J Environ Health.;38(6):401-4.
- ISO 14698-1:2003: Cleanrooms and associated controlled environments – Biocontamination control – Part 1: General principles and methods, International Standards Organization, Geneva, Switzerland.
- ISO 21149: 2017 – Cosmetics – Microbiology – Enumeration and Detection of aerobic mesophilic bacteria, International Standards Organization, Geneva, Switzerland.
- Sandle T. (2010) ‘The Media Kitchen: Preparation and Testing of Microbiological Culture Media’. In Sutton, S. (ed.): Laboratory Design: Establishing the Facility and Management Structure, Parenteral Drug Association, Bethesda, MD, United States, ISBN 1-933722-46-0, pp269-293
- Sandle T. (2014) Examination of the Order of Incubation for the Recovery of Bacteria and Fungi from Pharmaceutical Cleanrooms, International Journal of Pharmaceutical Compounding, 18 (3): 242 – 247.
- Sutton SV, Proud DW, Rachui S, Brannan DK. (2002) Validation of Microbial Recovery From Disinfectants. PDA J Pharm Sci Tech. 56(5): 255-266.
- Schiemann DA, Chatfield D. (1976) Evaluation of neutralizers in Rodac media for microbial recovery from disinfected floors, J Environ Health. 38(6):401-4.
- Winely C, Sexton K, Bobey D. (1998) Neutralization of beta-lactam antibiotics in an environmental monitoring medium, PDA J Pharm Sci Technol.;52(6):344-5.
- Sandle T. (2011) ‘Microbial recovery on settle plates in unidirectional airflow cabinets’, Clean Air and Containment Review, Issue 6, pp8-10.
- Engley FB, Dey BP. (1970) A universal neutralizing medium for antimicrobial chemicals. In Proceedings of the 56th Mid-year Meeting of the Chemical Specialties Manufacturers Association. New York, pp. 100–106.
- Sandle T. (2018) Microbiological Culture Media: A Complete Guide for Pharmaceutical and Healthcare Manufacturers, DHI / PDA, Bethesda, MD, USA.
- Anderson NL, Noble MA, Weissfeld AS, Zabransky RJ. (2005) Cumitech 3B, Quality Systems in the Clinical Microbiology Laboratory, Coordinating ed., Sewell DL, ASM Press, Washington, D.C.
- Bolton FJ, Coates D, Hutchinson DN. (1984) The ability of campylobacter media supplements to neutralize photochemically induced toxicity and hydrogen peroxide, J Appl Bacteriol.;56(1):151-7.
Biography
Dr Tim Sandle’s primary role is Head of Microbiology at Bio Products Laboratory, a sterile products manufacturer. In addition, he is a tutor with the School of Pharmacy and Pharmaceutical Sciences, University of Manchester, for the university’s pharmaceutical microbiology MSc course, and a longstanding committee member of the pharmaceutical microbiology society Pharmig.
The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- bi-monthly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Issue
Related topics
Aseptic Processing, Assays, Bioproduction, Clinical Development, Environmental Monitoring, QA/QC