First patient recruited in IMCgp100 uveal melanoma trial
Posted: 30 March 2016 | | 1 comment
The trial of IMCgp100 will include three Phase I escalation cohorts to determine the optimal dose for the Phase II study, which is expected to start in 2016…
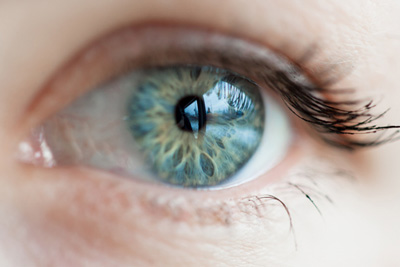

Immunocore has recruited the first patient into a Phase I monotherapy trial of its lead programme, IMCgp100, for the treatment of uveal melanoma.


Uveal melanoma, a rare disease in which cancer cells form in the tissues of the eye, comprises approximately 3% of all melanomas, and is the primary intraocular malignancy of the adult eye. There are currently no effective treatments on the market for this debilitating disease.
The trial will include three Phase I escalation cohorts to determine the optimal dose for the Phase II study, which is expected to start in 2016.
In January 2016, the US Food and Drug Administration (FDA) granted Orphan Drug Designation to IMCgp100 for the treatment of uveal melanoma. Orphan Drug status qualifies Immunocore for a number of development incentives to enable rapid progress in the clinical development of IMCgp100 in advanced uveal melanoma.
The Orphan Drug Designation programme provides orphan status to drugs and biologics, defined as those intended for the safe and effective treatment, diagnosis or prevention of rare diseases or disorders where the prevalence of the condition affects no more than 200,000 people in the US.
IMCgp100 has shown promising signs in uveal melanoma
Commenting on the news, Christina Coughlin, Chief Medical Officer of Immunocore, said: “Advanced uveal melanoma is a rare and devastating disease for which there are currently no effective treatment options. IMCgp100 has shown some promising signs of early clinical activity in this disease setting and could be an effective treatment for this group of patients. We are excited to be able to explore the activity of IMCgp100 in this clinical study in advanced uveal melanoma.”
IMCgp100 is Immunocore’s wholly-owned and most advanced ImmTAC (Immune mobilizing mTCR Against Cancer), currently in Phase I/IIa clinical trials for the treatment of late stage metastatic melanoma. Promising results from the Phase I/IIa clinical trial of IMCgp100 in advanced cutaneous and uveal melanoma patients demonstrated that responses were durable, with five objective responses to date, two of which are partial responders in patients with uveal melanoma. To date, more than 85 patients have been treated with IMCgp100.







I’m a uveal melanoma patient from the UK. This trial is of great interest to me as my prognostic biopsy resulted in me having an intermediate risk of metastatic disease. I will follow the progress of Sheila Levine, who is in the trial, via the eye cancer patients forum on facebook.
I wish it the greatest success!!