Cancer-fighting nano-robots programmed to seek and destroy tumours
Posted: 13 February 2018 | Dr Zara Kassam (European Pharmaceutical Review) | No comments yet
Study shows first applications of DNA origami for nanomedicine…
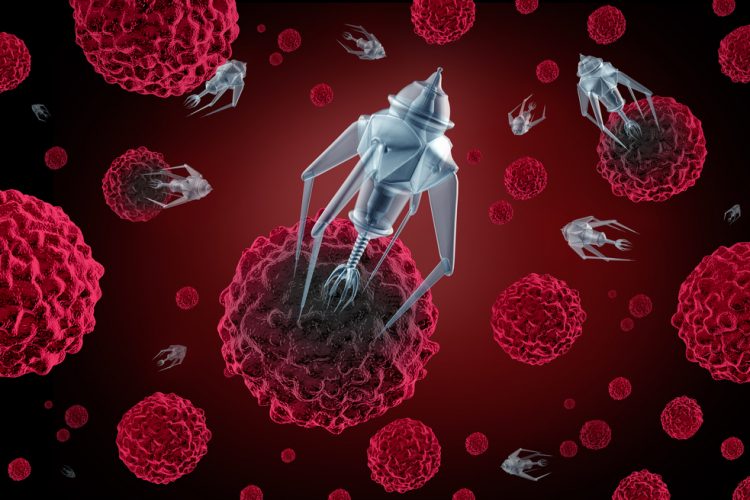

In a major advancement in nanomedicine, researchers have successfully programmed nanorobots to shrink tumours by cutting off their blood supply.
“We have developed the first fully autonomous, DNA robotic system for a very precise drug design and targeted cancer therapy,” said Hao Yan, director of the ASU Biodesign Institute’s Center for Molecular Design and Biomimetics and the Milton Glick Professor in the School of Molecular Sciences.
“Moreover, this technology is a strategy that can be used for many types of cancer, since all solid tumour-feeding blood vessels are essentially the same,” said Dr Yan.
To perform their study, the scientists took advantage of a well-known mouse tumour model, where human cancer cells are injected into a mouse to induce aggressive tumour growth. Once the tumour was growing, the nanorobots were deployed to come to the rescue. Each nanorobot is made from a flat, rectangular DNA origami sheet, 90 nanometers by 60 nanometers in size. A key blood-clotting enzyme, called thrombin, is attached to the surface.
Thrombin can block tumour blood flow by clotting the blood within the vessels that feed tumour growth, causing a sort of tumour mini-heart attack, and leading to tumour tissue death.
First, an average of four thrombin molecules was attached to a flat DNA scaffold. Next, the flat sheet was folded in on itself like a sheet of paper into a circle to make a hollow tube. They were injected with an IV into a mouse, then travelled throughout the bloodstream, homing in on the tumours.
The key to programming a nanorobot that only attacks a cancer cell was to include a special payload on its surface, called a DNA aptamer. The DNA aptamer could specifically target a protein, called nucleolin, that is made in high amounts only on the surface of tumour endothelial cells—and not found on the surface of healthy cells.
Once bound to the tumour blood vessel surface, the nanorobot was programmed, like the notorious Trojan horse, to deliver its unsuspecting drug cargo in the very heart of the tumour, exposing an enzyme called thrombin that is key to blood clotting.
The nanorobots worked fast, congregating in large numbers to quickly surround the tumour just hours after injection.
First and foremost, the team showed that the nanorobots were safe and effective in shrinking tumours.
“The nanorobot proved to be safe and immunologically inert for use in normal mice and, also in Bama miniature pigs, showing no detectable changes in normal blood coagulation or cell morphology,” said Yuliang Zhao, also a Professor at NCNST and lead scientist of the international collaborative team.
Most importantly, there was no evidence of the nanorobots spreading into the brain where it could cause unwanted side effects, such as a stroke.
“The nanorobots are decidedly safe in the normal tissues of mice and large animals,” said Guangjun Nie, another professor at the NCNST and a key member of the collaborative team.
The treatment blocked tumour blood supply and generated tumour tissue damage within 24 hours while having no effect on healthy tissues. After attacking tumours, most of the nanorobots were cleared and degraded from the body after 24 hours.
By two days, there was evidence of advanced thrombosis, and 3 days, thrombi in all tumour vessels were observed.
The key is to trigger thrombin only when it is inside tumour blood vessels. Also, in the melanoma mouse model, 3 out of 8 mice receiving the nanorobot therapy showed complete regression of the tumours. The median survival time more than doubled, extending from 20.5 to 45 days.
They also tried their system in a test of a primary mouse lung cancer model, which mimics the human clinical course of lung cancer patients. They showed shrinkage of tumour tissues after a 2-week treatment.
For Dr Yan, the important study milestone represents the end of the beginning for nanomedicine.
“The thrombin delivery DNA nanorobot constitutes a major advance in the application of DNA nanotechnology for cancer therapy,” said Yan. “In a melanoma mouse model, the nanorobot not only affected a primary tumour but also prevented the formation of metastasis, showing promising therapeutic potential.”
Dr Yan and his collaborators are now actively pursuing clinical partners to further develop this technology.
“I think we are much closer to real, practical medical applications of the technology,” said Dr Yan. “Combinations of different rationally designed nanorobots carrying various agents may help to accomplish the ultimate goal of cancer research: the eradication of solid tumours and vascularised metastases. Furthermore, the current strategy may be developed as a drug delivery platform for the treatment of other diseases by modification of the geometry of the nanostructures, the targeting groups and the loaded cargoes.”
“These nanorobots can be programmed to transport molecular payloads and cause on-site tumour blood supply blockages, which can lead to tissue death and shrink a tumour,” said Baoquan Ding, a professor at the NCNST, located in Beijing, China.
The successful demonstration of the technology, the first-of-its-kind study in mammals utilising breast cancer, melanoma, ovarian and lung cancer mouse models, has been published in the journal Nature Biotechnology.
Related topics
Anti-Cancer Therapeutics, Clinical Development, DNA, Nano-medicine, Technology
Related organisations
Arizona State University, National Center for Nanoscience and Technology