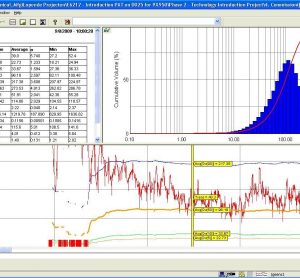
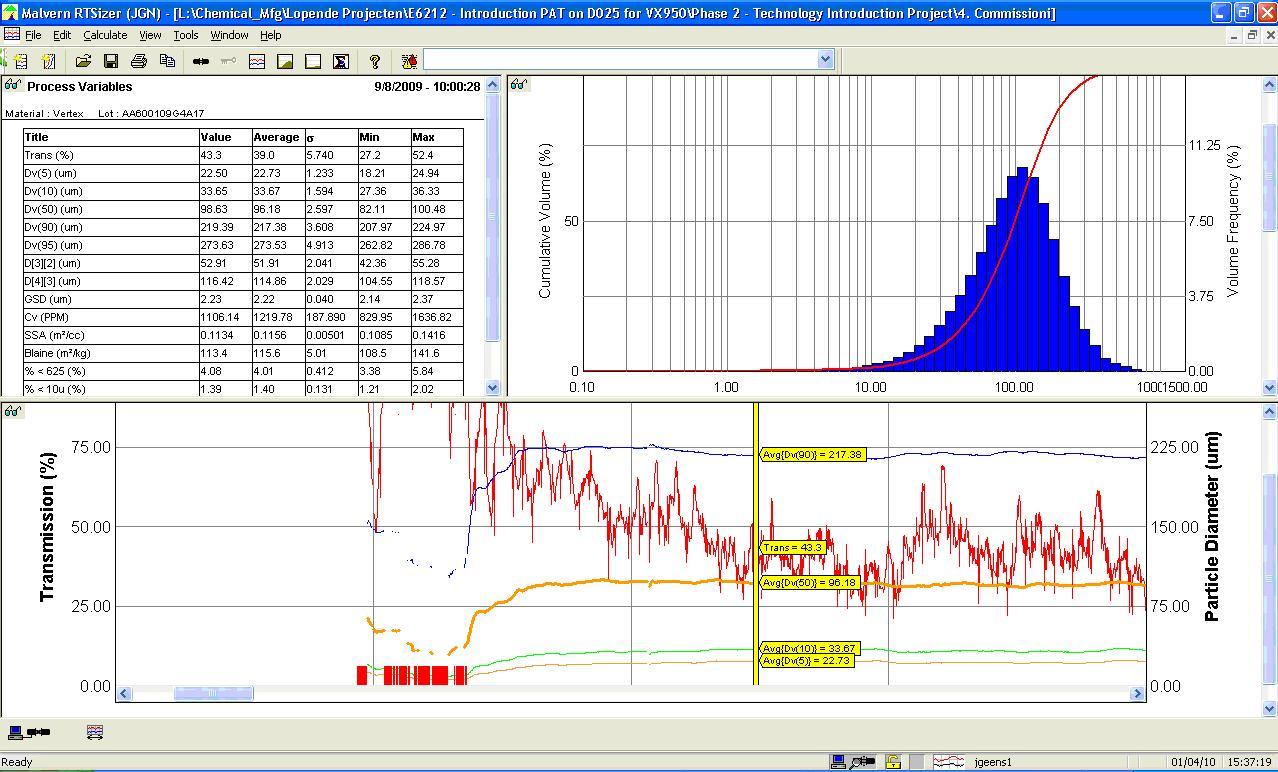
Spectroscopy In-Depth Focus 2016
In this Spectroscopy In-Depth Focus: The benefits of NIRS in…
In this Spectroscopy In-Depth Focus: The benefits of NIRS in monitoring and controlling a crystallisation process; Pharmaceutical applications of terahertz spectroscopy and imaging; and much more...