Zika Virus: a public emergency of international concern
Posted: 4 February 2016 | | 2 comments
The Zika virus has taken hold in South America. Its possible link to microcephaly in newborn babies and neurological conditions has led the World Health Organization to declare a Public Health Emergency of International Concern. Here we discuss the virus, countermeasures and the race for a vaccine.

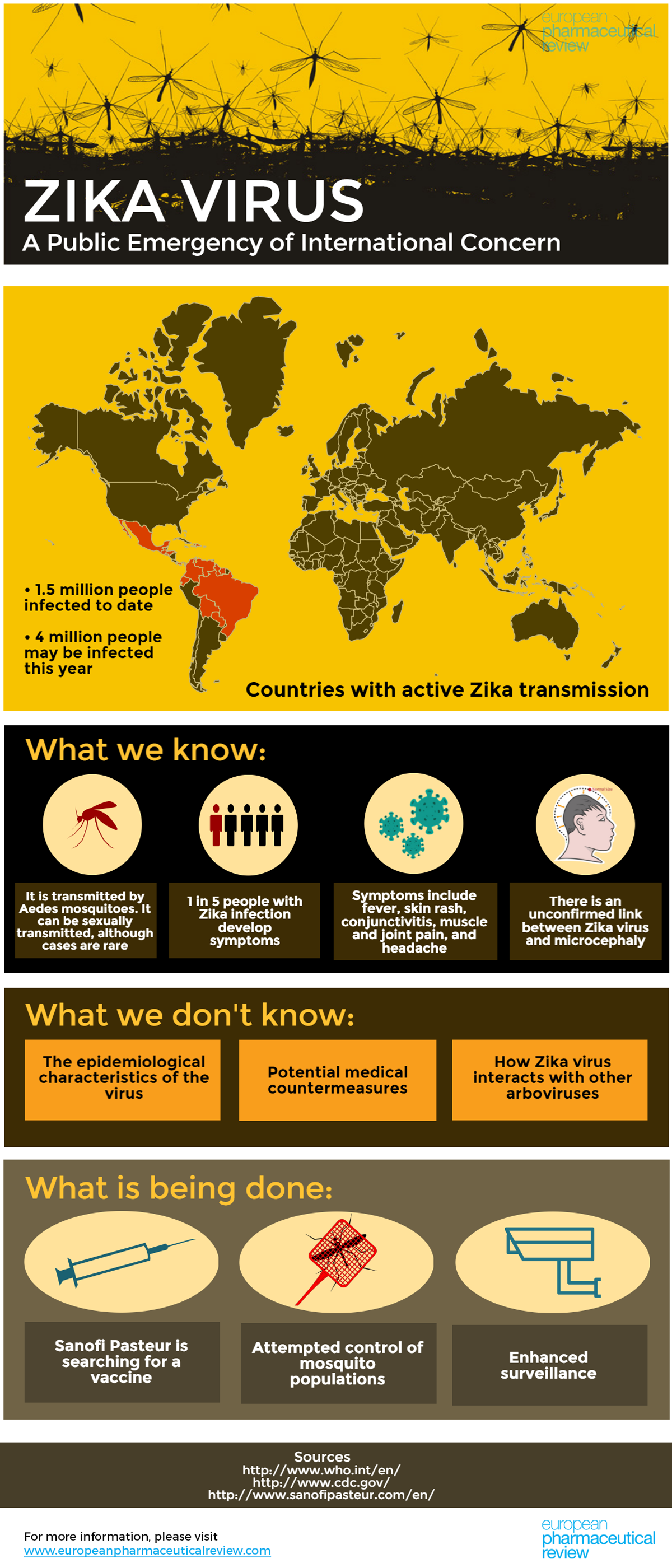
The World Health Organization (WHO) has estimated that up to 1.5 million in Brazil have been infected with the Zika virus since the outbreak began last year. WHO says that up to 4 million people could be infected this year.
But what is Zika?
The Zika virus, a mosquito-borne flavivirus related to the yellow fever, dengue and West Nile viruses, has taken hold in South America.
Very little is known at the moment and there is currently no vaccine available. The virus was first identified in Uganda in rhesus monkeys in 1947 and was then identified in humans in 1952. Since then there have been outbreaks recorded in Africa, the Americas, Asia and the Pacific.
We created an infographic to break it down for you:
So what is being done to tackle Zika virus?
After convening a panel of experts from around the world, WHO has declared that the microcephaly cases and other neurological disorders reported in Brazil constitutes a Public Health Emergency of International Concern. They have called for an international response to improve surveillance, the detection of infections, congenital malformations, and neurological complications, to intensify the control of mosquito populations, and to expedite the development of diagnostic tests and vaccines.
As there is no vaccine available, the WHO says the most important protective measures are the control of mosquito populations and the prevention of mosquito bites in at-risk individuals, especially pregnant women.
While WHO found no public health justification for restrictions on travel or trade to prevent the spread of Zika virus, the UK National Travel Health Network and Centre is advising those who are pregnant, those who are planning to become pregnant, or those with severe chronic illness or immune system disorders, to seek advice from a health professional before travel to areas where Zika virus outbreaks are currently reported.
In addition, the US Centres for Disease Control and Prevention (CDC) have issued travel recommendations for pregnant women to postpone travel to countries in Latin America and the Caribbean where Zika virus transmission is ongoing.
NHS Blood and Transplant have also introduced a 28 day blood donation deferral for people looking to donate blood who have travelled to countries where the Zika virus is endemic.
Race for a vaccine
Sanofi Pasteur, a leader in vaccine research, has said that it is going to launch an R&D development programme for a Zika virus vaccine.
Driving the new project, Nicholas Jackson, Global Head of Research, Sanofi Pasteur, told European Pharmaceutical Review:
“It is critical for regional and global collaborative efforts to rapidly move forward. We hope to leverage our existing expertise, technologies and infrastructure for our recently licensed dengue vaccine against the Zika virus to rapidly advance a vaccine candidate.”


Dr Nicholas Jackson will be driving the new project for Sanofi Pasteur
Sanofi Pasteur leads the vaccine field for viruses in the same family as Zika virus, with licensed vaccines against Yellow Fever, Japanese Encephalitis and Dengue.
Importantly, the company says its expertise and established R&D and industrial infrastructure for the newly licensed vaccine for dengue, Dengvaxia, can be rapidly leveraged to help understand the spread of Zika virus and potentially speed identification of a vaccine candidate for further clinical development.
Other pharmaceutical companies will also likely follow suit in hunting for a vaccine.
In addition, the Medical Research Council has launched a £1 million rapid response funding initiative to provide novel, critical and timely insights into the nature of the risk posed by the Zika virus and potential avenues for its management or prevention.
Current measures
The incubation period is not clear, but WHO says it is likely to be a few days. While the infection is spread by Aedes aegypti mosquito, there have been rare cases where the the virus has been sexually transmitted, including one recent case in Texas.
Roughly 80% of those infected with the virus do not develop any symptoms, making spread of the virus difficult to track. For those who do develop symptoms, they are usually mild and include fever, skin rashes, conjunctivitis, muscle and joint pain, malaise, and headache. These symptoms last for 2-7 days and can be treated with common pain and fever medicines, rest and plenty of water.
However, during a large outbreak in French Polynesia in 2013 and in the current outbreak in Brazil, there have been reports of an unusual increase in Guillain-Barré syndrome – a condition where the body’s immune system attacks parts of the nervous system. Investigations are being carried out to discover if the increase in cases of the syndrome can be linked to Zika.
In terms of where the virus could spread to, researchers have used environmental data to predict where the Zika-carrying Aedes aegypti mosquitoes can establish themselves. They conclude that Chile and Canada will likely be the only places in the Americas not at risk of local epidemics.
Unconfirmed risks
The outbreak in South America has coincided with a rise in cases of microcephaly in newborn babies. Since October, there have been more than 3,500 reported cases in Brazil (the country had fewer than 150 cases in 2014). On 12 January, the MOH Brazil confirmed that two miscarriages and two infants born at term (37-42 weeks) who died 24 hours after birth, tested positive for the Zika virus. The mothers of these babies had a history of rash and fever during pregnancy.
These findings, together with other evidence collected during 2015, reinforce the possibility of an association between exposure to Zika in pregnancy and congenital malformations. While investigations are ongoing to establish whether there is a link, the evidence is enough for WHO to state “that a causal relationship between Zika infection during pregnancy and microcephaly is strongly suspected”.
Other solutions?
Some researchers are looking to combat the virus at its source – in mosquitoes. The Eliminate Dengue Programme have explored how a bacterium that infects 60% of insects around the world may be used as a tool to combat the spread of dengue, Zika and similar mosquito-borne viruses. The bacterium, Wolbachia, is not naturally found in the Aedes aegypti mosquito. Researchers with Eliminate Dengue have found that when they infect mosquitoes with the bacteria in the lab, it prevents them from transmitting dengue, chikungunya and yellow fever.
“The Eliminate Dengue Programme is doing field experiments to see whether we will be able to replace wild-type, existing populations of mosquitoes with these Wolbachia-infected ones and whether it blocks dengue transmission” said Dr Matthew Aliota, a research scientist in the UW-Madison School of Veterinary Medicine (SVM) and member of the Eliminate Dengue group. “Now, we’re going to start looking at how that might be used for Zika virus control as well in South America.”
Others are claiming fish are the solution:
Source: AJ





![Thermo Fisher Scientific company office with large company logo in Silicon Valley, US [Credit: Michael Vi/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Thermo-Fisher-Scientific-400x187.jpg)



Dear Sir/Madam,
My personal belief is that the “measures and the actions” which have been and which are being put in place both to “fight” Zika virus (ZV) and to gain “new/further insight” into ZV infections’ ecology, epidemiology and biology make sense and appear to be more than appropriate.
Nevertheless, as a Veterinary Pathologist involved since years in the study of “infectious diseases” (including “West Nile virus infection” in horses) and also with a strong interest in “Veterinary and Comparative Neuropathology”, I think that characterizing the “viral neurotropism and neuropathogenicity” represents a “key issue” in the study of VK infection’s pathogenesis. This would be relevant not only for a thorough assessment of the “causal relationship(s)” between ZV infection and “microcephaly”, as well as “Guillain-Barrè syndrome” in humans, but also for the possibility (and the related value/significance) of determining the presence/occurrence of anti-ZV antibodies (also) within the cerebro-spinal fluid (CSF) from infected patients. As a matter of fact, alongside with the production of those detectable in serum, a parallel” production of “intrathecal” antibodies (detectable in CSF) may be expected in the course of infections caused by “neurotropic pathogens”, once the concerned biological “noxa” has reached and settled itself inside the “central nervous system” (CNS, brain and/or spinal cord), after crossing the “blood-brain barrier” or “invading” the host’s CNS by retrograde nerve transport. Therefore, evidence of anti-ZV antibodies within the CSF could be “synonimous” of “CNS (viral) infection”, a prerequisite for the subsequent development of “microcephaly” and, likely, also of “Guillain-Barrè syndrome”.
Another “key-issue” related to those specified above is the “time frame” within which “embrionic/foetal infection” occurs, should this be true (also) with ZV (in relation to the occurrence of “microcephaly”), as previously reported in the case of “West Nile virus infection”. This is a “crucial issue”, provided that the “developing organism” is not equally susceptible to “teratogenic stimuli” (which are not restricted to those of “infectious nature”) throughout its life inside the maternal uterus: “embrios”, in fact, are much more susceptible to “teratogenic insults” than “foetuses”.
Furthermore, should ZV infection take place during “embrionic/foetal development”, it would be also important to investigate whether the virus is able to infect the embrionic/foetal “thymus”, a pathogenetic feature which could provide it with an “additional capability” of escaping host’s immune defense mechanisms by means of a process/phenomenon known as “immune tolerance”.
Finally, in a very recent “Perspective” published in “New England Journal of Medicine (NEJM)”, concern has been drawn on the possibility that, apart from/alongside with its “conventional” invertebrate vector represented by Aedes aegypti, other mosquitoes such as A. albopictus could become “efficient” ZV vectors. A similar “scenario” would likely “make things worse”, since A. albopictus is definitely more common than A. aegypti in the Northern as well as in the Western Hemisphere (with 32 among the 50 States from USA having detected its presence/occurrence, according to the aforementioned “NEJM Perspective”). In this respect, I would also like to point out that A. albopictus has been recently identified as an “alternate” vector for “Dirofilaria immitis”, a cardio-pulmonary nematode (worm) infecting dogs and cats, which is “conventionally” carried by (non-A. albopictus) Aedes, Anopheles and Culex mosquitoes. Furthermore, the challenging possibility that Wolbachia spp., a Gram-negative bacterium infecting 60% of insects around the world – but not A. aegypti, inside which it is not found under natural conditions – could be used as a “weapon” against the spread of Dengue, Chikungunya, Yellow Fever and Zika viruses, could well represent an extremely valuable option. However, since it has been proven that Wolbachia spp. may also infect D. immitis, the aforementioned cardio-pulmonary nematode, thereby eliciting an inflammatory response in affected cats and dogs, it would be absolutely important to investigate “whether and how” interfering with, or by means of, Wolbachia in the “ecology, epidemiology and evolution” of given arthropode-borne infections would “affect” not only the “mosquito vector” but also the “microorganism” carried inside it, be it a virus or a nematode (as in the case of D. immitis infection). Indeed, antimicrobial therapy against Wolbachia spp. has resulted in decreased microfilarial loads, inhibition of the development of larval worms, female worm infertility and reduced numbers of Wolbachia organisms. Consequently, at least in the concerned arthropode-borne infection’s “model”, the presence and the number of Wolbachia spp. organisms appears to be “functional” to a “canonical” development and progression of the disease process, quite differently from what seen in Wolbachia-challenged mosquitoes, in which the bacterium prevents them from transmitting Dengue, Chikungunya and Yellow Fever viruses. At the light of what above, this appears to be an extremely delicate “equilibrium”, made up by 4 different, mutually interacting “variables”, namely the “pathogen”, the “insect”, the “host” and the “environment”, with large plausibility existing about the possibility that the “interaction patterns” of the microorganism and Wolbachia may substantially diverge from those taking place between Wolbachia and the pathogen’s “vector”. Within this framework, one should consider that the beneficial effects toward the host of an “antimicrobial therapy” – such as that against Wolbachia in D. immitis-infected dogs – could be counteracted by the (putatively) detrimental effects toward the insect’s “intrinsic ability” to transmit the “microbial agent” carried inside it (and viceversa), a “scenario” which is made of even greater concern by the aforementioned possibility that other mosquitoes such as A. albopictus, which are far more common in “developed Countries”, could become “efficient” ZV vectors.
In conclusion, I believe that the above issues and concerns would deserve adequate attention on behalf of “Decision Makers”, together with “ad hoc” research efforts on behalf of the Scientific Community.
Giovanni DI GUARDO, DVM, Dipl. ECVP,
Scientific Editor,
“Research in Veterinary Science”,
Professor of “General Pathology and
Veterinary Pathophysiology”,
University of Teramo,
Faculty of Veterinary Medicine,
Località Piano d’Accio,
64100 – Teramo, Italy
(E-mail address: [email protected])
I am a little bit puzzled about the decision of the WHO. Chikungunya, a closely related virus caused some 540 000 cases in the Dominican Republic within 15 months and has spread to vast areas in America as well – and it is spreading still. An estimated number of some 360 mio. of Dengue cases (published in Nature) and thousands of deaths occur each year with some 1.6 mio. cases in 2015 in Brazil alone – and WHO never declared a PHEIC because of Dengue. Now Zika virus, an agent known since more than 60 years that usually doesn´t kill people pops up in Latin America – by the way transmitted by the same mosquito – and results in an PHEIC ?? I don´t want to play Zika down and diseases like microcephaly and GBS must be taken very seriously – but is this a balanced decision? Who are the drivers involved in the decision making process which resulted in ignoring Ebola in West Africa for far too long (a catastrophy WHO must be taken responsible for) and now pushing Zika like almost in panic?