J&J’s Tremfya secures two paediatric psoriasis approvals from FDA
Posted: 30 September 2025 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
The US FDA approvals make the drug the first IL-23 inhibitor to be licensed for two common skin conditions.
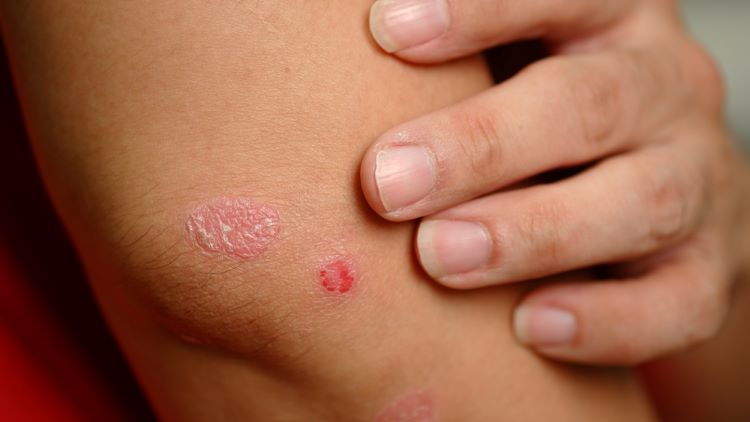

The Food and Drug Administration (FDA) has approved Johnson & Johnson’s Tremfya (guselkumab) to treat paediatric plaque psoriasis and active psoriatic arthritis, making it the first IL-23 inhibitor to gain such a licence in the US.
The subcutaneous injection monoclonal antibody is now indicated for children six years old and over who weigh at least 40kg with moderate to severe plaque psoriasis (PsO), and are candidates for systemic therapy or phototherapy, or who have active psoriatic arthritis (PsA).
Approval of Tremfya was based on data from the phase III PROTOSTAR study, presented at the 2025 AAD Annual Meeting.
“despite advancements in the treatment of paediatric plaque psoriasis and active psoriatic arthritis, there continues to be a significant gap in available therapies for these debilitating immune-mediated diseases”
At Week 16, 66 percent of moderate to severe plaque PsO patients attained skin clearance. This was compared to 16 percent of patients given placebo treatment. Furthermore, almost 40 percent of participants achieved complete clearance, versus just four percent who did so on placebo.
Additionally, the FDA’s approval was supported by evidence from pharmacokinetic extrapolation analyses from trials including the phase III VOYAGE 1 and 2 studies in adults with moderate to severe plaque PsO, as well as the DISCOVER 1 and 2 studies.
Significance of the approval
Dr Vimal Hasmukh Prajapati, Clinical Associate Professor, University of Calgary and study investigator, explained that “despite advancements in the treatment of paediatric plaque psoriasis and active psoriatic arthritis, there continues to be a significant gap in available therapies for these debilitating immune-mediated diseases that impact a child’s physical and emotional wellbeing during critical years”.
Therefore, authorisation of Tremfya represents “an important step forward” for patients, according to Brandee Pappalardo, PhD, Vice President, Medical Affairs, Dermatology & Rheumatology, Johnson & Johnson Innovative Medicine.
The FDA’s latest decision adds to the agency’s earlier authorisations of Tremfya in adults with moderate to severe plaque PsO in 2017 and active PsA in 2020.
The drug has also shown long-term promise for ulcerative colitis patients, as seen in phase III trials released in May.
Related topics
Big Pharma, Biologics, business news, Clinical Development, Clinical Trials, Data Analysis, Drug Development, Drug Markets, Drug Safety, Industry Insight, Regulation & Legislation, Research & Development (R&D), Therapeutics









