Positive top-line results for Ascendis Pharma’s TransCon Growth Hormone
Posted: 30 July 2015 |
Ascendis Pharma has announced positive top-line results from a study evaluating the safety and efficacy of TransCon Growth Hormone in children with GHD…

Ascendis Pharma has announced positive top-line results from a six-month Phase 2 study evaluating the safety and efficacy of once-weekly TransCon Growth Hormone in 53 treatment-naïve, pre-pubertal children with growth hormone deficiency (GHD).


GHD is a serious orphan disease affecting both children and adults. In children, GHD manifests with short stature, metabolic abnormalities, cognitive deficiencies and poor quality of life. Adult GHD is associated with premature mortality and neuropsychiatric-cognitive, cardiovascular, neuromuscular, metabolic and skeletal abnormalities. The global market for daily injections of human growth hormone was approximately $3 billion in 2014. There are currently no long-acting growth hormone treatment options available in the United States or Europe.
The Phase 2 paediatric study was conducted to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of TransCon Growth Hormone in treatment-naïve pre-pubertal children with growth hormone deficiency, or GHD, who meet internationally recognized diagnostic criteria for GHD. The study enrolled 53 patients into the treatment phase and was a 6-month study comparing three dose levels of TransCon Growth Hormone, administered once per week, to the active control Genotropin, administered as a daily injection.
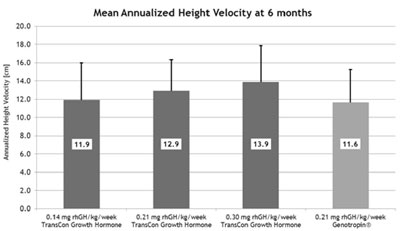
Mean annualised height velocities among the three dosing levels administered weekly ranged from 11.9 cm for the 0.14 mg/kg/week TransCon Growth Hormone dose to 13.9 cm for the 0.30 mg/kg/week TransCon Growth Hormone dose, which were comparable to 11.6 cm for the active comparator, daily injections of Genotropin at a 0.21 mg/kg/week dose.

“We are extremely pleased with the top-line results from our Phase 2 paediatric study and believe these data establish once-weekly TransCon Growth Hormone as the potential best-in-class long-acting human growth hormone program,” stated Jan Mikkelsen, President and Chief Executive Officer. “Most importantly, these results move us one step closer to fulfilling our goal of developing an innovative therapy that has the potential to improve the real-world treatment outcomes of patients with GHD.”
Once-weekly TransCon Growth Hormone appears comparable to gold-standard daily therapies
Professor Pierre Chatelain, M.D., Coordinating Investigator of the Phase 2 paediatric study, former Chairman of the College of Paediatrics at the Université Claude Bernard Lyon 1, and Professor Emeritus of Paediatrics, stated, “I am highly encouraged by the mean annualised height velocity achieved by once-weekly TransCon Growth Hormone in the Phase 2 paediatric study. Based on these top-line data, once-weekly TransCon Growth Hormone appears comparable to gold-standard daily growth hormone therapies in terms of efficacy, safety and tolerability, with reduced injection frequency that may allow for improved patient compliance and treatment outcomes.”
Barbara Lippe, M.D., Professor Emerita of Paediatrics, David Geffen School of Medicine atUCLA and clinical advisor to Ascendis Pharma stated, “For children suffering from GHD, the goal of therapy is to restore safe and effective levels of growth hormone throughout the body so that the child has the potential to normalize metabolism and body composition and achieve a normal adult height. This Phase 2 study demonstrates that the TransCon prodrug releases unmodified growth hormone at peak and overall exposure levels that are comparable to daily growth hormone therapies. This preserves the same mode-of-action and distribution within the body as daily growth hormone therapies, for which safety and efficacy have been demonstrated over more than 25 years of clinical experience.”
Full data from the Phase 2 will be released in October 2015 at the annual meeting of the European Society for Paediatric Endocrinology.