FDA approves first-of-a-kind oral therapy for rare disease
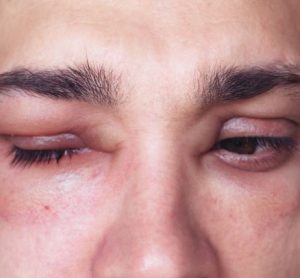
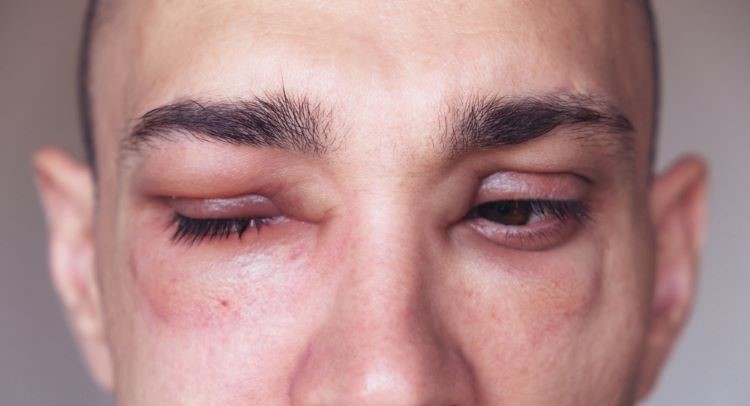
The US drug agency’s authorisation provides hereditary angioedema (HAE) patients…
The US drug agency’s authorisation provides hereditary angioedema (HAE) patients with the first new on-demand treatment in over ten years.