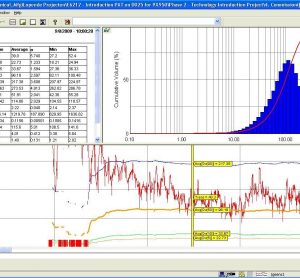
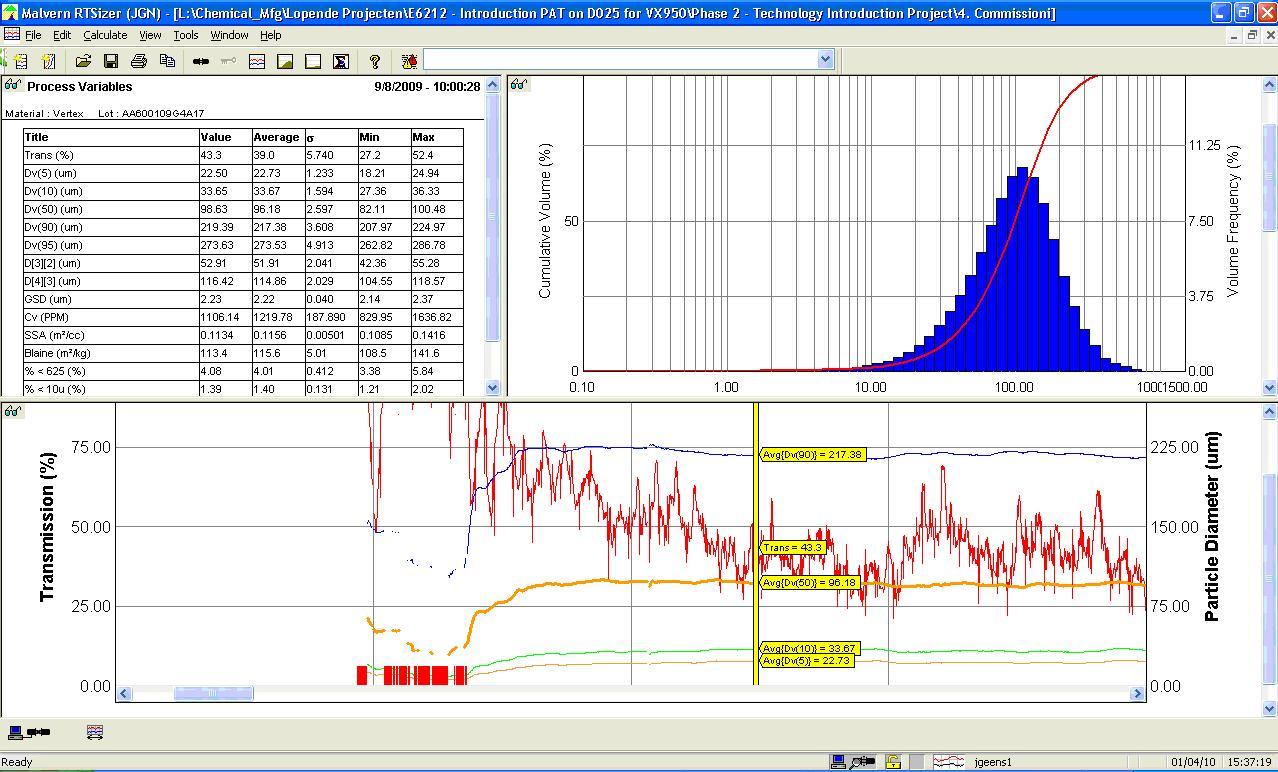
Process Analytical Technology (PAT): In-Depth Focus 2013
In this Process Analytical Technology (PAT) In-Depth Focus: Presenting a…
In this Process Analytical Technology (PAT) In-Depth Focus: Presenting a rational approach to QbD-based pharmaceutical development: A roller compaction case study, PAT for pharmaceutical spray drying, PAT Roundtable, Show Preview: IFPAC® 2014...