Lipid-based formulations: Their advantages in early development
Posted: 21 March 2017 | | No comments yet
Patheon’s Kaspar van den Dries shows the advantages of considering lipid formulation in early development to optimise a molecule’s developability…

An assessment of developability at the earliest stages of drug development can expedite candidate selection, facilitate go/no-go decisions, and move promising molecules into clinical trials more rapidly.
The developability of a molecule depends on a wide variety of chemical, biopharmaceutical and pharmaceutical factors. A thorough understanding of these elements is essential in de-risking development programs, and ensuring the financial resources and time involved are kept to a minimum.
Choice of formulation technology is an important step in optimising developability. This article examines the advantages of considering lipid formulation in early development and the potential of a structured screening and development approach to optimise a molecule’s developability by overcoming potential barriers related to lipid formulations.
General challenges with poor bioavailability
Pharmaceutical companies face many challenges throughout the lifecycle of a chemistry, manufacturing and controls (CMC) development project, including limitations in bioavailability, which can make it hard to obtain meaningful toxicology and/or clinical data (Figure 1).
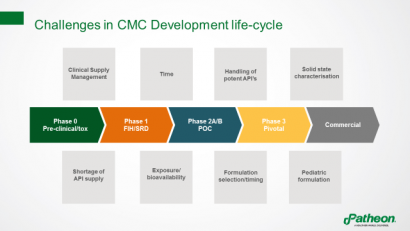

Figure 1: Challenges in the lifecycle of CMC development
Almost 70% of compounds are considered as Biopharmaceutics Classification System (BCS) Class II, with low solubility and high permeability, hindering their ability to enter the circulation and produce desired therapeutic effects (Figure 2). Improving solubility and bioavailability is a multifaceted challenge.
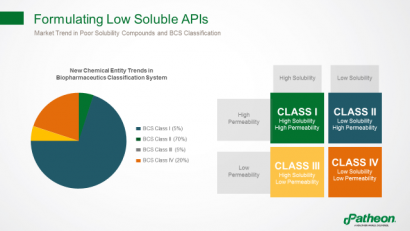

Figure 2: Formulating poorly soluble active pharmaceutical ingredients (APIs)
There may be a temptation for companies with one or two preferred technologies to try to force a new molecule into these. However, there are many technologies to choose from, some with an assumed probability of success of 20% to 40%. Considering multiple solutions in parallel is the most promising way to address solubility limitations, since testing technologies in sequence can be expensive and time-consuming, and may provide unexpected results. A solubility enhancement formulation platform can provide a useful structure for this process, including technology selection, formulation design, process development and production of clinical trials materials.
Paradoxically, although many new drug applications (NDAs) over the past decade have involved poorly soluble compounds, formulation technologies were used to overcome bioavailability issues in only 15% of these molecules (Figure 3). Instead, other means were used, including increasing drug loading and using more API to achieve the same systemic exposure.
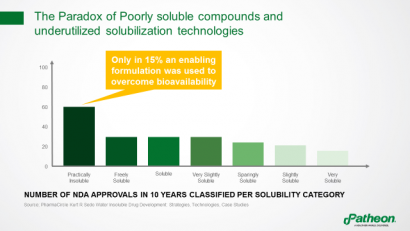

Figure 3: The paradox of poorly soluble compounds and underutilised solubilisation technologies
Among solubilisation technologies, lipid formulations have been most widely used in approved drug products since 1980, followed by dispersions (Figure 4). Nanocrystals and amorphous drugs can also help overcome bioavailability issues.
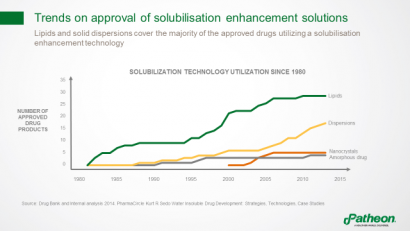

Figure 4: Trends in use of solubiliation enhancement in approved drugs
Pharmaceutical companies are often reluctant to switch formulation once early development has been completed. There may be several reasons for this:
- A simple formulation such as a tablet or a capsule is frequently used in early development, so any switch would entail repeat pharmacokinetic testing, with potential to delay development,
- In early development proof of concept is top of mind, and CMC costs are not a major focus of attention, and
- While there are many available solubilization technologies, these are complex and many in the industry are not fully aware of all their options.
Bioavailability: A major cause of product failures
Several studies have shown that bioavailability is an important reason for product failures, with the pharmacokinetic profile more often to blame than toxicity or lack of efficacy (Figure 5).
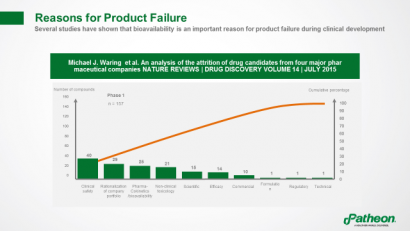

Figure 5: Reasons for product failure1
Technologies to improve bioavailability
Multiple technologies are available to enhance solubility and overcome bioavailability issues (Figure 6). One example is particle size reduction, which is used if dissolution rate is the limiting factor. Solubilisation can be achieved using lipid systems, microemulsions, self-emulsifying drug delivery systems (SEDDs), co-solvents or solubility enhancers such as cyclodextrins. Another option is to use solid dispersions, based on an amorphous drug that is stabilised using a polymer, and then processed by spray drying or hot melt extrusion.
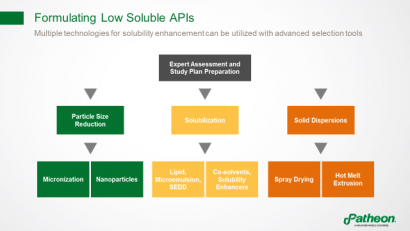

Figure 6: Using advanced selection tools to identify appropriate formulations
Lipids and spray dry dispersions are most commonly used, accounting for 80% of marketed solubilised drugs since 1980, as both have wide applicability. Lipid systems can be formulated as softgels, liquid-filled hard shell capsules or oral solutions (Panel).
Panel: Manufacturing options for lipid formulations
Oral solutions
· Consider taste and stability
· Ideal for larger concentrations/animal studies
Liquid-filled hard shell capsule
· Require banding or overcoating to seal
· Shell compatibility needs to be investigated
· Ideal for small volumes/early PK studies
Softgel capsules
· Can be developed at small scale
· Versatile/easy to scale-up
· Ideal for commercialisation
Integrated modeling platform
Based on studies performed in the late 1990s, the developability of molecules can be assessed using the Lipinski Rule of Five (Ro5; Figure 7).


Figure 7: The Lipinski Rule of Five2
However, many molecules currently in clinical development do not fit within the boundaries of the classical Ro5.3 In addition, the Ro5 does not provide criteria for selection of the most appropriate formulation to optimise developability. Today, more advanced tools are available that assist in technology selection, helping with bioavailability challenges and formulation design.4
For lipid-based systems, information about potential food effects can assist in formulation decisions. Since more than 70% of BCS Class II compounds exhibit positive food effects, interacting with dietary lipids in the gastrointestinal tract, it makes sense to consider lipid systems to improve bioavailability and absorption. Figure 8 illustrates an appropriate approach for lipid formulation screening, taking account of the API’s lipid solubility and compatibility, emulsion dispersibility in biorelevant media and the effect of lipid digestion. The screening approach is set up so that minimal amounts of API are required to achieve a robust formulation design.


Figure 8: Screening methodology for lipid formulations
In conclusion, poor bioavailability remains an important cause of product failure. Lipid formulations – which are currently underutilised – have promise for overcoming bioavailability issues with many APIs. Pharmaceutical companies should consider working with an experienced partner to apply scalable and science-based approaches at the earliest stage of development to boost each molecule’s chance of success.


References
- Waring MJ, Arrowsmith J, Leach AR, et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov. 2015;14(7):475-486.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3-26.
- Doak BC, Over B, Giordanetto F, Kihlberg J. Oral druggable space beyond the rule of 5: insights from drugs and clinical candidates. Chem Biol. 2014;21(9):1115-1142.
- Wessel M CM, Konagurthu S, Reynolds T. How Broadening the Analysis of CompoundFactors Allows for Predictive Solubility Solutions. Pharmaceutical Online, available at https://wwwpharmaceuticalonlinecom/doc/how-broadening-the-analysis-of-compound-factors-allows-for-predictive-solubility-solutions-0001. June 6, 2016.



