Targeted therapy in metastatic melanoma
Posted: 28 February 2012 |
During the last few years, significant improvements in the treatment of metastatic melanoma were reported, targeting molecules involved in the pathogenesis of melanoma. Different clinical trials were able to prove a prolonged overall survival by introducing new therapeutic agents. Hereby an imunomodulating therapy with the anti-CTLA-4 antibody ipilimumab has been established. Other promising treatment possibilities include targeted therapies for melanoma patients showing certain activating mutations in their tumour cells, e.g. BRAF V600 mutations and their selective inhibition by vemurafenib or the inhibition of the c-Kit receptor by drugs such as imatinib mesylate. This review will provide a brief overview of the latest therapeutic strategies and recent achievements in treating metastatic melanoma, as well as discuss the arising problems with resistance mechanisms to selective therapies. It will also highlight future approaches to combine specific treatments in an attempt to individualise melanoma treatment for every patient with the best possible efficacy and outcome…

During the last few years, significant improvements in the treatment of metastatic melanoma were reported, targeting molecules involved in the pathogenesis of melanoma. Different clinical trials were able to prove a prolonged overall survival by introducing new therapeutic agents. Hereby an imunomodulating therapy with the anti-CTLA-4 antibody ipilimumab has been established. Other promising treatment possibilities include targeted therapies for melanoma patients showing certain activating mutations in their tumour cells, e.g. BRAF V600 mutations and their selective inhibition by vemurafenib or the inhibition of the c-Kit receptor by drugs such as imatinib mesylate. This review will provide a brief overview of the latest therapeutic strategies and recent achievements in treating metastatic melanoma, as well as discuss the arising problems with resistance mechanisms to selective therapies. It will also highlight future approaches to combine specific treatments in an attempt to individualise melanoma treatment for every patient with the best possible efficacy and outcome.
Melanoma skin cancer is quickly becoming one of the most common and dangerous cancer types in Europe and the United States. It ranks as the fifth most common newly diagnosed cancer in men and holds the seventh place in women1. While early detected melanoma can be cured surgically, the treatment of metastasised melanoma remains a major challenge.
Until recently, the treatment of metastatic melanoma was performed by chemotherapeutic regimes such as dacarbazine (DTIC) monochemotherapy or different polychemotherapeutic regimes with response rates of less than 40 per cent and no advantage in overall survival. The median overall survival of metastatic melanoma patients treated with DTIC was reported to be six to eight months2,3. Recent data from molecular research points to different mechanisms of melanoma pathogenesis. Theoretically, every activating mutation or deletion in the identified signal transduction pathway (Figure 1) regulating cell survival and proliferation in the healthy melanocyte provides a substrate for targeted therapy.
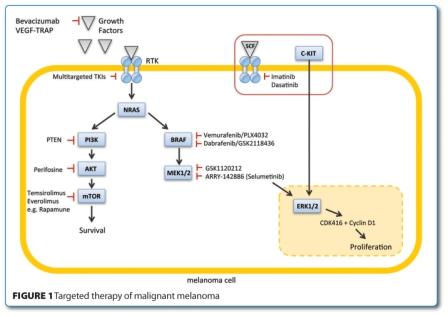

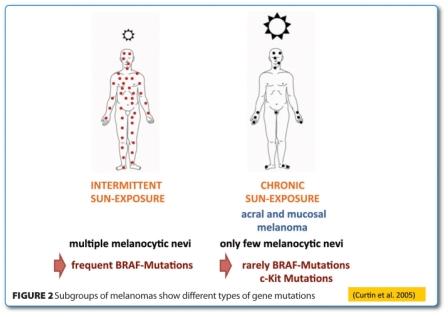
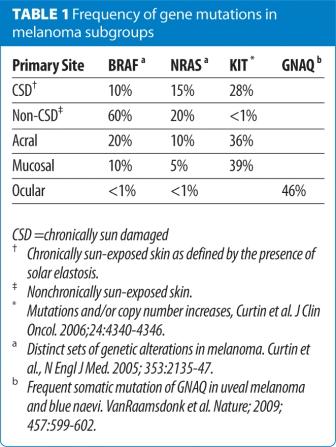
In clinical practice, however, things are far more complex. The early described NRAS gene mutations, one of the antecedent points of the pathway, has been detected in approximately 20 per cent of melanoma patients4, but no efficacious therapy has resulted as of yet5. Most patients with melanoma (90 per cent) report at least intermittent sun exposure. In 60 per cent of the melanomas, which develop in non-chronically sun-damaged skin, activating mutations of the BRAF gene are seen6 (Figure 2). These mutations have been discovered as a causing agent for melanoma in over 50 per cent of the patients but also give rise to other cancer types, such as colorectal or papillary thyroid cancer in around eight per cent of the cases7. In melanomas developed in chronically sun damaged skin as well as in acral or mucosal melanomas, mutations in the c-Kit gene are found in more than 30 per cent of the patients (Table 1 and Figure 2). And the extremely rare infestation of ocular melanoma exhibits activating mutations of the GNAQ gene in 46 per cent of the affected cases. Therefore, different molecular causes of melanoma require different therapeutic approaches and further investigation of all dysregulating mutations and melanoma subtypes will be required to tailor the targeting more specifically8. This review is to give a brief overview of the new therapeutic strategies and recent progress in the treatment of metastatic melanoma.



Targeted therapy of BRAF
Ninety per cent of the mutations in the BRAF gene in melanoma cells are located in the activation segment V600E, where glutamic acid is substituted for valine. Looking more thoroughly at patients with activating mutations in the BRAF gene, some interesting aspects become clear. BRAF gene mutated patients are younger than the average melanoma patient, more women are afflicted and they are unfortunately more prone to brain metastases. V600K BRAF mutation was shown to correlate with a more aggressive phenotype with more brain metastases and a shorter disease free and overall survival9. In a recent clinical trial with the BRAF-inhibitor vemurafenib (PLX4032) (BRIM-3 study), 675 BRAF V600Emutated, metastatic and previously untreated melanoma patients were examined and vemurafenib was compared to the standard treatment with dacarbazine (DTIC) chemo – therapy10. Patients in the vemurafenib arm showed a reduction in risk of death and tumour progression of more than 60 per cent, respectively 70 per cent. Overall survival as well as progression free survival of these previously untreated BRAF mutated melanoma patients was prolonged. The positive effect of vemurafenib could not be seen in BRAF wildtype11. The most common adverse effects included cutaneous events such as rash, arthralgia, fatigue and photosensitive skin reactions. In 38 per cent of the patients, a dose reduction was necessary. Eighteen per cent of patients developed a squamous cell carcinoma or keratoakanthoma.
Resistance mechanisms
Ever since targeted therapies were introduced in clinical trials, resistance mechanisms of melanoma cancer cells appeared, underlining the high aggressivity of these cancer cells and their high potential of adaptability to overcome selective inhibiton. Johannessen et al, for example, reported that COT/MAP3K8 drives resistance to RAF inhibition via MAP kinase pathway reactivation12. Long-term treatment of BRAF V600E mutant melanoma with a selective and specific BRAF inhibitor can lead to an RAF kinase switch (ARAF or CRAF) with subsequent cross-resistance to selective BRAF inhibitors13. Furthermore, higher activity of PI3K/AKT and enhanced IGF-1R were reported in chronically treated melanoma cells. Therefore, concomitant MEK and PI3K/AKT inhibition to overcome or prevent resistance to selective BRAF inhibitors is proposed13. Nazarian et al emphasise that melanomas chronically treated with vemurafenib acquire resistance to BRAF inhibition by RTK or NRAS upregulation14. Thus, targeting these two key points could help to prevent or overcome melanoma resistance to selective BRAF-inhibitors.
MEK Inhibition
Targeting the mitogen-activated protein kinase MEK leads to the inhibition of ERK1/2, the only substrate of this kinase. Since MEK plays a role in signal transduction downstream of BRAF, it may constitute another potential candidate for targeted interference when inhibition of BRAF shows resistance. In a preclinical study, Solit et al showed that MEK inhibition is more effective in BRAF gene mutated melanoma cells15. The two MEK inhibitors selumetinib and GSK1120212 currently tested in clinical trials are administered orally daily and are well tolerated. Selumetinib (AZD6244/ARRY-142886) is an ATP-dependent uncompetitive selective inhibitor of MEK 1/2. Kirkwood and colleagues recently published results from a phase II study with 200 metastasised melanoma patients, where temzolomide chemotherapy and selumetinib monotherapy were compared16. Apparently, no significant difference in progression free survival was found, but further investigation will be required to draw more detailed conclusions. GSK1120212, a selective allosteric MEK 1/2 inhibitor, is presently under investigation in clinical trials as well. Falchook and colleagues presented data from over 200 patients in which 90 per cent exhibited an extended metastatic disease and almost every included patient had been treated with different therapies before17. Moreover, nearly half of all patients had received treatment for brain metastases. Patients with BRAF mutated melanomas yielded response rates of over 40 per cent as compared to those with wild-type, who showed response rates of <10 per cent only. In the majority of patients, at least a partial response was seen. Interestingly, two patients who had developed resistance to the BRAF inhibitor vemurafenib remained stable under MEK inhibition with GSK1120212. The oral treatment with two milligrams was welltolerated and showed no grade 4 adverse events. Rash, fatigue, diarrhoea and reduced appetite were reported as more common adverse effects. A central serous retinopathy was seen at a daily dose of three milligrams18.
Targeted therapy of c-kit
C-Kit gene mutations can be detected in subgroups of melanomas such as melanomas on chronically sun-damaged skin or in acral and mucosal melanomas19. The most common c-Kit mutation is L576P which seems to be targeted specifically with the tyrosin kinase inhibitor dasatinib20. Kluger et al. investigated the efficacy of dasatinib in metastasised melanoma and discovered partial responses even in patients without the presence of a c-Kit mutation21. Other clinical trials report response rates of 50 – 80 per cent in patients with c-Kit gene mutated melanoma8,22. Hodi and colleagues showed a clinically significant response to imatinib in a female patient with metastasised anal mucosal melanoma23. She was treated with oral imatinib mesylate 400 milligram daily and staging performed four weeks after treatment initiation revealed a reduction of tumour masses in the pelvis and the left kidney. In-vitro tests with c-Kit gene mutated mucosal melanoma cells showed inhibition not only in the MAPK but also in the PI3/Akt pathway after administration of imatinib. This tyrosine kinase inhibitor had effects on proliferation and induced apoptosis in c-Kit gene mutated melanoma cells. A still unresolved and clinically critical problem, however, is the reduced ability of c-Kit inhibitors to pass the blood brain barrier. Disease progression under c-Kit inhibitors was detected especially in the central nervous system24.
Targeting CTLA-4 on T-lymphocytes
Ipilimumab is a human monoclonal antibody (IgG) directed against the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). CTLA-4 regulates T-cell activation and antitumor immunity is promoted upon its inhibition. In a phase III study, 676 patients with metastatic melanoma were observed for four years25. They were randomised in three groups in a 3:1:1 ratio. One group received the vaccine glycoprotein 100 (gp100) plus ipilimumab, group two was given gp100 as a single therapy and the third group was treated with ipilimumab alone. Ipilimumab was administered four times every three weeks intravenously at a dose of 3 mg/kg body weight. Results showed a superior efficacy of the T-cell potentiator independently from coadministration with gp100. Median overall survival was 10 months. Prior to ipilimumab and the targeted BRAF inhibitors, the one year survival rate of stage 3 and 4 melanoma patients was reported to be approximately 15 per cent. Under ipilimumab treatment, however, it rose to 45 per cent and increased survival of up to 25 per cent of the patients after the second year. The adverse effects under ipilimumab reached grade three to four in 10 – 15 per cent of the treated cases. As expected, 60 per cent of effects were immune-related and affected the skin or gastrointestinal tract. In general, diarrhoea was the most common reported side effect. Inflammation of the pituitary gland occurred in eight cases. All adverse effects required early recognition and an adequate treatment (corticosteroids, endocrine hormone substitution or infliximab). It is worth mentioning that the anti-cancer effect of the immunotherapy occurred after a latency period of approximately three months. Recently, another clinical trial introduced new perspectives in treating melanoma patients using combination therapies, where an improved overall survival after the admini – stration of dacarbazine plus ipilimumab has been reported26.
Other new therapy options in metastatic melanoma
Tasisulam (LY573636 sodium) is an anti – proliferative and cytotoxic drug that induces the mitochondrial cell death pathway27. In a phase II study conducted with 68 metastasised melanoma patients who had received chemotherapy before, tasisulam was administered intravenously every 21 days with a targeted dose Cmax of 420 μg/mL28 and yielded measurable antitumor activity and in some cases tumour mass reduction, respectively stable disease. The drug has now entered a phase III clinical trial to examine its superiority over the use of paclitaxel.
Sorafenib is a non-selective multikinase RAF-inhibitor for which a placebo-controlled phase III trial with metastasised melanoma patients under treatment with carboplatin and paclitaxel was conducted. One group was additionally administered a placebo, the other group received sorafenib. Results failed to prove any beneficial effect of sorafenib29. Similarly, negative results were reported by other groups30. On the contrary, data from a combination therapy of sorafenib with PI3/Akt/mTOR inhibitor showed promising results with this dual treatment regimen31. Notably, a monotherapy of each of the agents did not lead to any improvement. The PI3/Akt pathway is mobilised in about 70 per cent of all melanomas and subsequent cell cycle progression, cell proliferation and angiogenesis in melanoma cells are activated. Tumours with these cells are highly resistant to chemotherapies.
Another therapeutic strategy for advanced melanoma was recently derived from the treatment of advanced renal cell cancer32. Fifty three patients were treated with a combination therapy of the antiangiogenic VEGF inhibitor bevacizumab and the mTOR inhibitor everolimus. In renal cell cancer, treatment was efficient and well tolerated. Thirteen per cent of the metastasised melanoma patients showed a significant response, 53 per cent featured an improvement of tumour staging. The progression free survival was four months.
Since the NOTCH signalling pathway was identified as an important promoter of cancer metastases including in melanoma, its potential for a novel melanoma target therapy is presently under evaluation. Preclinical in-vitro studies on mice melanoma cell lines using RO4929097, a selective gamma secretase inhibitor that inhibits the NOTCH signalling pathway, have been performed and showed a growth impairment of pre-existing tumours as well as an inhibition of tumour formation in the animals33.
Outlook
Novel targeted therapeutic approaches for melanoma skin cancer offer improving survival rates, better quality of life and new hope for patients with a very poor prognosis when metastasised. Of these, the T-lymphocyteassociated antigen 4 (CTLA-4) antibody ipilimumab and the BRAF V600E selective inhibitor vemurafenib currently play the most prominent role. However, many obstacles still need to be overcome along the way. One is the occurrence of resistance mechanisms to selective inhibitors, where attempts are presently made to improve their control using combination therapies, e.g. chemotherapy with immunotherapy, immunotherapy with selective inhibitors of target points in the MAPK pathway (e.g. ipilimumab co-administered with vemurafenib) or multikinase inhibitors combined with selective agents. Many clinical trials have shown failure of single agent treatment, while combinations of the same substances lead to significant improvement of progression free survival or even tumour regression. At present, it seems to be crucial to boost the future identification of all specific mutations of the activating genes to warrant individualised therapies at an early point in time. Nonetheless, the most important and critical steps in any patient setting remain advocating melanoma prevention which especially includes informing the general public about the risks of UV radiation, performing a thorough clinical exam of the skin in an attempt to detect early stages of melanoma that can be cured surgically, as well as calling for regular skin screening exams adapting the latest clinical guidelines for higher-risk patients.
About the authors
Jochen Utikal is Head of the Skin Cancer Unit of the German Cancer Research Center in Heidelberg. Additionally, he is the senior consultant of the Skin Tumour Center Mannheim of the Ruprecht-Karl University Heidelberg. Prior to this, he was a scientist and research associate at the Massachusetts General Hospital Cancer Center and the Harvard Stem Cell Institute in Boston where he worked in the laboratory of Dr. Konrad Hochedlinger. His research is focused on similarities of stem cells with melanoma cells as well as on translational medical aspects of skin cancer research. He is a principal investigator of numerous promising phase I-IV clinical trials on skin cancers. He has published over 50 publications in international peer-reviewed scientific journals. For his research, he was awarded the Hella Bühler Prize, the Egon-Macher Award and the Fleur-Hiege Memorial Award.
Janina Staub is a clinical resident in dermatooncology at the Skin Tumour Center Mannheim of the University of Heidelberg and a postdoctoral researcher of the Skin Cancer Unit of the German Cancer Research Center in Heidelberg. Prior to this, she was a research associate at the Department of Physiology and Pathophysiology at the University Clinic of Heidelberg, working on vasculogenesis in the ischemic mouse brain in the laboratory of Dr. Hugo Marti. Her research and clinical interests include skin cancers, especially melanoma, where she is involved in several phase I-IV clinical trials as subinvestigator, as well as in trials on pain therapy in dermatooncology. In addition, she serves on the interdisciplinary tumour board of the University Medical Center Mannheim.
References
- Jemal, A., Siegel, R., Xu, J. and Ward, E. Cancer statistics, 2010. CA Cancer J Clin. 60: 277-300
- Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, Panageas KS, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999; 17:2745-51
- Bhatia S, Tykodi SS, Thompson JA Treatment of metastatic melanoma: an overview. Oncology. 2009; 23:488-96
- Padua RA, Barrass NC, Currie GA Activation of N-ras in a human melanoma cell line. Mol Cell Biol. 1985; 5:582-5
- Gajewski TK, Johnson J, Linette G, Bucher C, Blaskovich M, Sebti S, Haluska F. Phase II study of the farnesyltransferase inhibitor R115777 in advanced melanoma: CALGB 500104. J Clin Oncol. 2006; 24(18S) ND
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005; 353:2135-47
- Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417:949–954
- Handolias D, Hamilton, AL, Salemi R, Tan A, Moodie K, Kerr L, Dobrovic A and McArthur GA. Clinical responses observed with imatinib or sorafenib in melanoma patients expressing mutations in KIT. Brit J Cancer. 2010; 102:1219-1223
- El-Osta H, Falchook G, Tsimberidou A, Hong D, Naing A, Kim K, Wen S, Janku F, Kurzrock R. BRAF mutations in advanced cancers: clinical characteristics and outcomes. PLoS One. 2011; 6:e25806. Epub 2011 Oct 19
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011; 364:2507-16
- Bollag G, Hirth P, Tsai J et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010; 467:596–599
- Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010; 468:968-72
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, Santiago-Walker AE, Letrero R, D’Andrea K, Pushparajan A, Hayden JE, Brown KD, Laquerre S, McArthur GA, Sosman JA, Nathanson KL, Herlyn M. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010; 18:683-95
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to BRAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010; 468:973-7
- Solit, D.B., Garraway, L.A., Pratilas, C.A., Sawai, A., Getz, G., Basso, A. and Ye, Q. BRAF mutation predicts sensitivity to MEK inhibition. Nature 2006; 439:358-362.
- Kirkwood JM, Bastholt L, Robert C et al. Phase II, Open- Label, Randomized Trial of the MEK 1/2 Inhibitor Selumetinib as Monotherapy versus Temozolomide in Patients with Advanced Melanoma. Clin Cancer Res, November 2011, Article ahead of print (a)
- Falchook, G., Infante, J.R., Fecher, L.A., Gordon, M.S., Vogelzang, N.J., DeMarini, D.J. et al. The oral MEK1/2 inhibitor GSK1120212 demonstrates early efficacy signals. Paper presented at European Society of Medical Oncology October 8_12 2010, Milan, Italy
- Infante, J.R., Fecher, L.A., Nallapareddy, S., Gordon, M.S., Flaherty, K.T., Cox, D.S. et al. Safety and efficacy results from the first-in-human study of the oral MEK 1/2 inhibitor GSK1120212. J Clin Oncol 2010; 28:15S, Abstract 2503
- Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006; 24:4340-6
- Woodman SE, Trent JC, Stemke-Hale K, Lazar AJ, Pricl S, Pavan GM, Fermeglia M, Gopal YN, Yang D, Podoloff DA, Ivan D, Kim KB, Papadopoulos N, Hwu P, Mills GB, Davies MA. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther. 2009; 8:2079-85.
- Kluger, H. M., Dudek, A. Z., McCann, C. Ritacco, J., Southard, N., Jilaveanu, L. B., Molinaro, A., Sznol, M. A phase 2 trial of dasatinib in advanced melanoma. Cancer 2011;117:2202–8.
- Lutzky J, Bauer J, Bastian BC. Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res. 2008; 21: 492–3.
- Hodi FS, Friedlander P, Corless CL, Heinrich MC, Mac Rae S, Kruse A, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008; 26:2046–51.
- Sznol M. Molecular markers of response to treatment for melanoma. Cancer J. 2011; 17:127-33.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363:711-23.
- Robert C, Thomas L, Bondarenko I, O’Day S, M DJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011; 364:2517-26.
- Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011; 16:5-24.
- Kirkwood JM, Gonzalez R, Reintgen D, Clingan PR, McWilliams RR, de Alwis DP, Zimmermann A, Brown MP, Ilaria RL Jr, Millward MJ. A phase 2 study of tasisulam sodium (LY573636 sodium) as second-line treatment for patients with unresectable or metastatic melanoma. Cancer. 2011 Mar 31. doi: 10.1002/cncr.26068. [Epub ahead of print] (b)
- Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel assecond-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009; 27:2823-30.
- Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma:a Phase II randomised discontinuation trial analysis. Brit J Cancer. 2006; 95:581-6.
- Sánchez-Hernández I, Baquero P, Calleros L, Chiloeches A. Dual inhibition of (V600E) BRAF and the PI3K/AKT/mTOR pathway cooperates to induce apoptosis in melanoma cells through a MEKindependent mechanism. Cancer Lett. 2012; 314:244-55.
- Hainsworth JD, Infante JR, Spigel DR, Peyton JD, Thompson DS, Lane CM, Clark BL, Rubin MS, Trent DF, Burris HA 3rd. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma: a phase 2 trial of the Sarah Cannon Oncology Research Consortium. Cancer. 2010; 116:4122-9.
- Huynh C, Poliseno L, Segura MF, Medicherla R, Haimovic A, Menendez S, Shang S, Pavlick A, Shao Y, Darvishian F, Boylan JF, Osman I, Hernando E. The novel gamma secretase inhibitor RO4929097 reduces the tumour initiating potential of melanoma. PLoS One. 2011; 6(9): e25264.




