Why it’s high time for psychedelic drug development
Posted: 25 May 2021 | Hannah Balfour (European Pharmaceutical Review) | No comments yet
Here, Dr Matthew McMurray and Dr J Andrew Jones of Miami University explain why psychedelic drugs are attracting R&D as potential treatments for neuropsychiatric and degenerative disorders and how their discovery of an in vivo production technique could enable further advancement.


Psychedelic drugs are becoming an increasingly popular area of research and drug development. A market research report estimates that the sector could be worth more than $10 billion by 2027, growing at a compound annual growth rate (CAGR) of 12.36 percent from its value of $4.75 billion in 2020.1 According to this and other reports, the first US Food and Drug Administration (FDA)-approved psychedelic drug, Janssen-Cilag International NV’s Spravato (esketamine) nasal spray for treatment-resistant depression, authorised in the US on 5 March 2019 and in the EU and UK later the same year, is anticipated to help promote growth in the market.
Psychedelics, also known as hallucinogens, include a range of compounds – some extracted from plants or fungi and others made synthetically, that are able to induce hallucinations if administered in sufficient quantities. The most common psychedelic substances include ketamine, psilocybin (the active substance in Psilocybe cubensis – colloquially known as ‘Magic Mushrooms’), ibogaine, lysergic acid diethylamide (LSD), dimethyltryptamine (DMT) and 3,4-Methylenedioxymethamphetamine (MDMA).
To understand more about why pharma is investing in these drugs and how their recent discovery about the production of psilocybin could benefit the market, European Pharmaceutical Review’s Hannah Balfour interviewed Miami University’s Dr Matthew McMurray, Assistant Professor in the Department of Psychology, and Dr J Andrew Jones, Assistant Professor in the Department of Chemical, Paper and Biomedical Engineering.
Why are psychedelics attracting so much R&D?
Psychedelic drugs are being evaluated in a range of serious neuropsychiatric and neurodegenerative disorders, including major depressive disorder (MDD), bipolar and anxiety disorders such as post-traumatic stress disorder (PTSD), as well as Alzheimer’s and Parkinson’s disease (AD and PD, respectively). The prevalence of these conditions is growing, as is the population of those who are resistant to currently available treatments, and so novel drugs such as psychedelics are becoming more widely studied.
Dr McMurray added that, particularly for psychiatric applications, current treatments have several other limitations, including long onset periods that may require severely ill patients being treated for two weeks before evidence of therapeutic efficacy or serious side effects. “As such, there is a huge push in the pharmaceutical development industry to develop new medications that are more effective and can treat these diseases in a broader population. That is really where this interest in novel antidepressants, antipsychotics and anti-PTSD medications like psilocybin and other psychedelics is taking off.
“Much of this research started with ketamine, following some really promising clinical trials conducted roughly five years ago. Since then, the field has expanded beyond ketamine into other compounds, such as those we are developing and testing based on tryptamines. These have garnered significant clinical interest primarily due to several clinical trials wherein they demonstrated high efficacy with very low side effects in many people with debilitating diseases, like PTSD, depression or anxiety.”2
What are tryptamines and how do they work?
Tryptamines, such as psilocybin, are natural compounds or designer drugs that originate from the decarboxylation of tryptophan3 and produce hallucinations through agonism of the 5-hydroxytryptamine (5-HT) 2α serotonin receptors. It is thought that their anti-depressive and anti-anxiety effects are mediated through the same pathway.
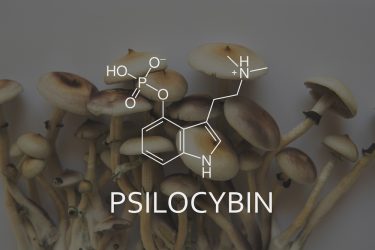

Dr McMurray explained: “Tryptamines are thought to modulate the serotonergic system, just like many available anti-depressants, but they target a very different aspect of that system. Specifically, the 5-HT2A receptors, which are different than the re-uptake transporters where the selective serotonin re-uptake inhibitors (SSRIs) like Prozac (fluoxetine) typically work. Since they target a specific receptor, instead of globally increasing serotonin throughout the brain, they are a potentially more targeted treatment for these disorders, reducing side effects such as serotonin syndrome. Another benefit is that they are naturally derived, not synthetic.”
He also explained that, because tryptamines target specific receptors, they should deliver the same therapeutic benefit – restructuring the serotonergic synaptic processes within the brain – without the debilitating withdrawal effects seen with SSRIs. In fact, Dr McMurray said that recent pre-clinical data from their lab suggests that a single dose of the tryptamine compounds they are developing may be effective in many people, where a typical SSRI like Prozac takes 15 to 20 days of treatment to reach early therapeutic efficacy. “Obviously, that is a much better clinical scenario because you may not have to keep treating people over and over; instead you could treat them once and send them on their way,” he said.
Considerations when developing psychedelic drugs
Tryptamines and the entourage effect
Beyond psilocybin, Dr McMurray and Dr Jones, and their corporate partner PsyBio Therapeutics Inc, are also developing other tryptamines, including norbaeocystin, which are yet to be studied in clinical trials. They are also researching how different tryptamines may interact and, thus, how combining them might enhance their beneficial effects, enabling a smaller therapeutic dose to be administered, or reduce such side effects as hallucinations.
This synergistic interaction between two or more different molecules when they are co-administered is known as the entourage effect and has been well studied in cannabinoids. According to Dr McMurray, because both cannabinoids and tryptamines are naturally derived products, the plants that produce them (Cannabis sativa and Psilocybe cubensis, respectively) also produce many similar compounds. For instance, Cannabaceae produce both cannabidiol (CBD) and tetrahydrocannabinol (THC). Similarly, in P. cubensis, psilocybin is produced alongside numerous other tryptamines, including norbaeocystin, baeocystin and aeruginascin .
In both cases these other compounds interact when they are present in the brain to induce a certain effect, such as the euphoria or ‘high’ associated with marijuana or hallucinations associated with magic mushrooms. By studying the effects of each compound, it is possible to identify those that may have a beneficial effect when combined, or those that induce the unwanted side effects that can then be omitted from future formulations. For instance, with cannabinoids, it has been shown that CBD is beneficial while THC induces the high; as such, THC is omitted from many formulations and there is a huge market for CBD products. The team is currently in the process of researching tryptamines to ascertain their individual effects and ascertain those that have positive therapeutic effects and those that have potentially negative hallucinogenic effects, as is the case with psilocybin when administered alone.
Regulatory restrictions
While regulation may have slowed down their development historically, the newfound success of tryptamines and other psychedelic drugs in neuropsychiatric and neurodegenerative indications is now encouraging new players to the market”
When questioned about the challenges surrounding psychedelic drug development, Dr Jones explained that there are two key concerns: regulation and production. He stated that, because psychedelic compounds are regulated in nearly every country worldwide, the basic science that typically initiates major therapeutic discoveries is lacking. “Because governments around the world have effectively been shutting down research or making it very difficult to perform for the last 70 years, we do not know as much about these classes of drugs as we would have otherwise. Essentially, we are at the early stages of this research, which is exciting because we have been making many new discoveries since starting to work with these compounds.”
He added, however, that the regulatory environment is still restrictive, since laboratories must be licenced by the Drug Enforcement Administration (DEA) in the US and similar agencies in other countries in order to study them. “Because they have been shown to be somewhat effective, there is now a bigger motivation to go through all that paperwork… and now there is a snowball effect, where the more people start to work and identify applications, the more it incentivises others to work in this space. So, we are slowly overcoming the regulatory challenge.” He also noted that talk about de-criminalising the use of magic mushrooms in certain US states, as with cannabis, could facilitate further scientific discovery.
Producing psilocybin
The other challenge Dr Jones highlighted was that, due to limited use and research, there is also a lack of knowledge about how to effectively synthesise and purify hallucinogenic compounds, as well as a deficiency in the basic chemical understanding of how these processes should work. PsyBio is a spin-out company, set up by Dr Jones and colleagues to develop a discovery he made about how to produce pharmaceutical grade psilocybin and other tryptamines in vivo using bacteria.4
“In my lab we are not only developing a completely new paradigm in how we produce these compounds, using biology instead of chemistry, but also having to develop some of the core downstream processing, manufacturing and purification steps that have just not been established for this class of compounds because they have been under-studied for so long,” explained Dr Jones.

He continued that his patent-pending discovery – that Escherichia coli can be metabolically engineered to sustainably produce psilocybin – overcomes one of the key hurdles that drug makers face when attempting to turn naturally occurring psychoactive compounds into medicines: variability.
“Traditionally, if you wanted to produce psilocybin, you would grow some mushrooms or find some illicit way to source these… The problem is that every batch of mushrooms that a farmer grows contains a different amount of these metabolites (tryptamines). Even if they are genetically the same, or grown on the same block, mushroom to mushroom you can get wildly different metabolite profiles. Additionally, mushrooms are difficult to produce and require special environments and humidity that must be controlled; they cannot just grow in rows like corn or soybeans, and they can also sometimes die for no apparent reason.” Consequently, he said, traditional mushroom farming is not suitable to meet pharma’s tryptamine production needs.
To overcome this, his team developed their novel synthesis technique. However, it was complicated by the fact that psilocybin and other tryptamines are naturally phosphorylated – stabilised with attached phosphate groups – when produced in mushrooms, which is a fundamental biological process, but difficult to achieve through chemistry. According to Dr Jones, to achieve this effect, the chemical synthesis process becomes extremely arduous, requiring several steps and intermediate purifications, as well as expensive palladium catalysts and several unpleasant chemicals to get a low yield of a particularly pricey product.
Dr Jones said that instead, in 2017 the biosynthetic pathway for tryptamines in mushrooms was discovered outside of his lab and it was demonstrated that purified enzymes could perform it in vitro, his lab then decided to test whether bacteria could perform the same process. Dr Jones explained: “By taking the DNA from the mushroom that encodes its ability to make this product and putting it in E. coli we were able to integrate the process into the native metabolism of the bacteria, which naturally produce tryptophan – the structure on which tryptamines are based.”
The team leveraged multiple genetic optimisation techniques to prompt the E. coli to overproduce psilocybin; these resulted in a 32-fold improvement in psilocybin titre. They then scaled up production using a bioreactor and, according to Dr Jones, by tweaking the operational parameters to determine the optimum fermentation conditions for the genetically superior organisms, they were able to further improve upon the production of psilocybin. Ultimately, they reached a production titre of 1.16 g/L of psilocybin in a fed-batch fermentation study; a near 500-fold increase from where they started when they first began experimenting with the process in bacteria.4
While we are continuing to develop and improve our processes, we are also starting work on downstream purification and other essential processes to speed up development”
Dr Jones added that this is the highest psilocybin titre achieved to date from a recombinant organism and that on metrics such as rate of production and yield from the substrate they put in, it exceeds every other technology. “As a result, my lab is now the first and only lab that can produce pharmaceutical-grade psilocybin in bacteria at industrially relevant production titres,” said Dr Jones, who added that this is a significant step toward demonstrating the feasibility of industrial production of biologically derived psilocybin.
He continued: “While we are continuing to develop and improve our processes, we are also starting work on downstream purification and other essential processes to speed up development and support ongoing clinical trials. This is what I am excited about. We are seeing this transition of science from the lab into potential clinical applications, with discussions about preparing for Investigational New Drug (IND) applications, in the span of 12 months.”
According to Dr McMurray and Dr Jones, not only is their method faster and cheaper than all other methods of tryptamine production, it is also greener and cleaner, requiring none of the hazardous products used in the traditional chemical synthesis methods. Dr Jones commented: “My cells grow in water-based media supplemented with sugar and salts. I like to think that you can pretty much stick your hand into anything in my lab and it is not going to hurt you. I promote life in my lab, as opposed to organic chemistry labs, where you have to be careful what you touch.”
Conclusions
While regulation may have slowed down their development historically, the newfound success of tryptamines and other psychedelic drugs in neuropsychiatric and neurodegenerative indications is now encouraging new players to the market and this, according to Dr McMurray and Dr Jones, has had a snowball effect on the number of developments made. Additionally, the discovery of a novel in vivo production method and associated research into developing downstream bioproduction and purification steps is a “significant step toward demonstrating the feasibility of industrial production of biologically derived psilocybin” and could further promote their development and adoption as a therapeutic option.
References
- Psychedelic Drugs Market, By Drugs (LSD, Ecstasy, Phencyclidine, GHB, Ketamine, Ayahuasca, Psilocybin), Route of Administration (Oral, Injectable, Inhalation), Distribution Channel, End-Users, Application and Geography – Global Forecast to 2026 [Internet]. MarketDigits; 2020. Available from: https://www.researchandmarkets.com/reports/5240207/psychedelic-drugs-market…
- Vargas A, Luís Â, Barroso M, Gallardo E, Pereira L. Psilocybin as a New Approach to Treat Depression and Anxiety in the Context of Life-Threatening Diseases—A Systematic Review and Meta-Analysis of Clinical Trials. Biomedicines [Internet]. 2020 [cited 4 May 2021];8(9):331. Available from: https://doi.org/10.3390/biomedicines8090331
- Fattore L, Zanda MT. Chapter 29 – Novel Psychoactive Substances: A New Behavioral and Mental Health Threat. Addictive Substances and Neurological Disease [Internet]. Academic Press; 2017 [cited 26 April 2021]. p. 341-353. Available from: https://www.sciencedirect.com/science/article/
- Adams A, Kaplan N, Wei Z, Brinton J, Monnier C, Enacopol A, et al. In vivo production of psilocybin in E. coli. Metabolic Engineering [Internet]. 2019 [cited 28 April 2021];56:111-119. Available from: https://doi.org/10.1016/j.ymben.2019.09.009
Related topics
Active Pharmaceutical Ingredient (API), Biopharmaceuticals, Bioprocessing, Bioproduction, Cannabinoids, Clinical Development, Clinical Trials, Drug Development, Drug Manufacturing, Drug Safety, Drug Targets, Therapeutics
Related organisations
Janssen-Cilag International NV, Miami University, US Food and Drug Administration (FDA)
Related drugs
cannabidiol (CBD), Cannabinoids, LSD, MDMA, psilocybin, Spravato (esketamine), tetrahydrocannabinol (THC)
Related diseases & conditions
Alzheimer’s disease, Anxiety, bipolar disorder, Parkinson's disease, post-traumatic stress disorder, PTSD








