PAT for the product lifecycle of a biopharmaceutical product
Posted: 25 January 2007 | | No comments yet
The biopharmaceuticals industry has undergone a number of revolutions in the past decade, not least the variety of ‘omics’ that focus on high throughput technologies to identify new product targets and can rapidly characterise those targets at small scale. However, it has been widely recognised that the technology used in the manufacturing of these in new products has lagged behind. In many respects, Process Analytical Technology (PAT) is a new ‘omics’ for biopharmaceutical manufacture.
The biopharmaceuticals industry has undergone a number of revolutions in the past decade, not least the variety of ‘omics’ that focus on high throughput technologies to identify new product targets and can rapidly characterise those targets at small scale. However, it has been widely recognised that the technology used in the manufacturing of these in new products has lagged behind. In many respects, Process Analytical Technology (PAT) is a new ‘omics’ for biopharmaceutical manufacture.
The biopharmaceuticals industry has undergone a number of revolutions in the past decade, not least the variety of ‘omics’ that focus on high throughput technologies to identify new product targets and can rapidly characterise those targets at small scale. However, it has been widely recognised that the technology used in the manufacturing of these in new products has lagged behind. In many respects, Process Analytical Technology (PAT) is a new ‘omics’ for biopharmaceutical manufacture.
The FDA has recognised that imposed regulatory burdens had resulted in industry being slow to accept and implement new technology that would have improved processes by reducing process and product variability, cost and the patient risks associated with the variability. The suggestions put forward by the FDA provides a framework that puts together disparate subject areas under the one aim, to mitigate risk by applying sound science and engineering principles over the life cycle of pharmaceutical development (FDA panel).
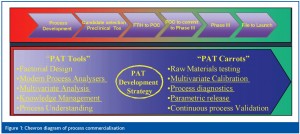
The use of the component ‘PAT Tools’ are not new and the ‘PAT Carrots’ represent perceived drivers that will push PAT implementation forward (Figure 1). Apart from identifying the need to implement new technologies in the production environment, the FDA recognises the need to carry out PAT supporting activities throughout the development lifecycle. These supporting activities build up a ‘knowledge base’ around the product that forms the basis for process understanding. Although the advantages of process analytical tools in manufacturing are clearly laid out in the FDA guidance, much of the emphasis to date has been on implementing PAT within existing licensed commercial processes.
Design space
The idea of a design space is a central concept to the new PAT regime and much has been accomplished to define a common understanding of what design space means (see draft ICH Q8 definition in box)


The concept of design space entails establishing a multivariate knowledge space that links critical process variables with critical to quality variables (CQA). This design space can be most efficiently mapped out using a variety of Design of Experiment (DoE) techniques, which minimise the number of experiments required. The description of design space suggests a single space in which a process operates. However, the DoE based design space is two spaces, (Factor and Response spaces) mapped onto each other by way of a mathematical transformation. A number of issues should be considered prior to planning a design space DoE:
1. Unit Operations
Many processes are a series of linked unit operations that progress raw materials through intermediate to final product in a batch wise manner. Some unit operations would be expected to have larger effects than others on the critical quality attributes. Formal risk assessment using Failure Mode Effect Analysis (FMEA) and other risk analysis tools allow users to rate each unit operation for their relative effect on quality (response) variables. Process capability indices on critical quality attributes (CQA) can be used as a useful metric to measure current state and improvements derived from PAT implementation.
2. Factor (Variable) and response selection
Variables that define the factor space need to be selected objectively. Formal risk assessment using Failure Mode Effect Analysis (FMEA) and other risk analysis tools allow users to rate variables for their effect on quality (response) variables. The assessment can be based on existing process knowledge. The assessment can also be aided with multivariate analysis (MVA) of historical process data and DoE variable screening designs.
3. Real time data
Although some process dynamics can be captured using DoE, DoE is unsuitable for analysis of real time data. Multivariate analysis tools such as PCA and Batch-PLS are more suited to this role. When DoE is used in conjunction with DoE, this powerful combination allows the user to identify critical process variables and model their relationship with CQAs including real time process dynamics effects.
PAT integration into process development
What is yet to be addressed are practical strategies to construct a process design space that spans the lifecycle of the product. Biopharmaceutical manufacturing processes usually consist of a set of unit operations connected in series, processing raw materials through intermediates to final product in a batch-wise manner. Each unit operation is described by a number of measured variables that are either controlled or free to vary. Only some of these variables will be critical to final product quality. This batch mode of processing means that each unit operation is run independently from the preceding or following operations. The complexity of many processes and the number of processing steps means that initial analysis should be focused on single unit operations. With infinite resource, it may be possible to investigate the design space for all unit operations. In practice, it makes sense to focus on the unit operations that are understood to contribute to most of the product quality variability.
The approach that many biopharmaceutical development organisations use to bring a candidate to the clinic and commercial licence has a number of features that aid building a design space over the course of the product development lifecycle. Biopharmaceutical development organisations rely on a platform technology approach where successive products in the pipeline are ‘plugged into’ a ‘skeleton’ manufacturing process. For monoclonal antibodies, this process is illustrated in Figure 1.
Some variation in the unit operation sequence between manufacturers will occur, but the skeleton process is used to speed product to proof of concept studies of first time in human studies and enable high product throughput through the development cycle. High throughput process development is achieved, in part, by using a limited set of ‘generic’ host cell lines, expression vectors, growth and production media and buffers that are progressed through this skeleton process. In conjunction with common scale up and downstream processing equipment, forming an existing skeleton process, an initial assessment of unit operation and process variability can be determined. This would add to the knowledge gained from previous product.
A Meta – DoE analogy for building a design space over a product development lifecycle
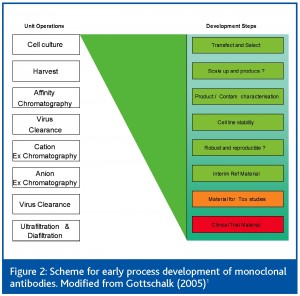
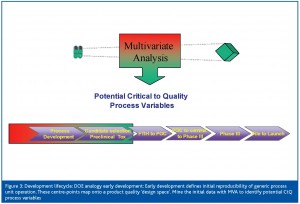
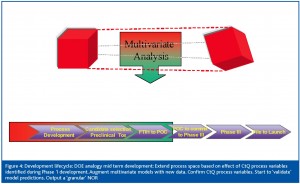
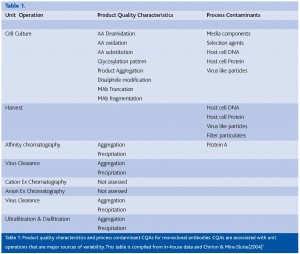
For the purposes of this paper, the upstream cell culture scale up and production stages will be used as an example. The cell culture steps are regarded2,3 to be key unit operations which affect CQA variability (Table 1). The mapping of a design space for a given product can be thought of in terms of a ‘meta’ DOE design through the process development lifecycle. At each Phase, (Figure 3, Figure 4, Figure 5 and Figure 6) combined use of DOE and Multivariate analysis captures process set-points, process dynamics of critical to quality (CtQ) process variables and their effect on CQA variability.
Stage 1: Early development (Figure 3)
A number of development steps shown in Figure 2 can provide data to form the basis of the design space. After cell line selection and process optimisation, multiple small scale batches are run to evaluate cell culture process robustness and reproducibility. Scale up and production batches’ use of manufacture material for toxicological studies and clinical trials provides additional data to assess reproducibility at a larger scale. These data acquired from early development defines the initial centre-points in the overall design space (Figure 3). At this stage there is little variation in the process factors and multivariate data analysis can be used to assess real time data to identify process variables that correlate with CQA variability. In conjunction with FMEA risk analysis and prior process knowledge, a panel of potential (CtQ) process variables can be identified.
Stage 2: Mid-term development (Figure 4)
Following first time in human studies (FTIH), further development of the process would involve expanding out the process space to a 3-level factorial space. The process variables identified as potential CtQ process variables in the preceding phases are used as factors in the design. The aim at this stage is to create a ‘granular’ normal operating range (NOR) and validate the variables as critical to quality variables. The real time data collected during this stage can be used to augment the multivariate models built during stage 1.
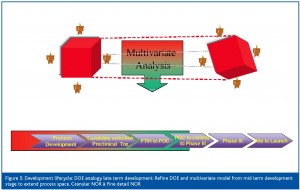
Stage 3: Late term development (Figure 5)
Stage 3 studies would further expand this space to extend the design space to a 5 level factorial space. The aim at this stage is to refine the detail within the design space and start to identify potential process failure regions. Again, the dynamics of the process are captured using multivariate analysis.
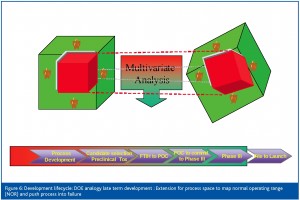
Stage 4: Late term development (Figure 6)
Stage 4 studies involve further expansion of the design space to increase the fine detail and push the design space into process failure. The aim is to refine understanding of where the limits of the design space are.
Summary
The strategy described above is intended to be applied to products where a skeleton process is already in place. Application of the strategy over multiple products will lead to enhanced understanding of key variables that affect product quality. As yet, a number of issues need to be reviewed within this strategy. Data acquisition and structured storage is critical to passing between each stage. In a case where a cell line change is required, there is little published data to compare the effect of a different cell line, derived from the same host cell bank on multivariate process trajectories. Similarly, there is little published work on multivariate techniques for process scale up and comparability for biopharmaceutical processes.







References
- Gottschalk U (2005), BioPharm International 18(6) : pp42
- Chirion & Mire-Sluise, (2004) Nature Biotech 22(11) : pp1383
- Webber, K. (2005) J. Proc Anal Technol, 2(4) :pp12







