QbD for biopharmaceutical product lifecycle management
Posted: 21 April 2025 | Busol Park (Samsung Biologics), Youngsun Kim (Samsung Biologics) | No comments yet
Youngsun Kim and Busol Park from Samsung Biologics detail the benefits of using a Quality-by-design approach for biologics manufacturing.


Quality-by-design (QbD) is a systemic risk-management strategy that facilitates the production of safe and consistent drugs. As pharma and biotech companies move towards more complex medicines, it is critical they have contract development and manufacturing organisation (CDMO) partners who can implement QbD principles while maintaining the accelerated timelines required for competitive business.
Process development, characterisation and validation with QbD
The lifecycle of a biopharmaceutical product takes a significant investment of resources. Given that early inconsistencies in a new drug can erode trust in novel products, pharma and biotech companies require rigorous manufacturing standards that can support their products from the early development stages through commercialisation and beyond.
For this reason, biopharma companies and their CDMO partners are increasingly using the QbD approach that leverages statistics to mitigate risks and optimise processes.
QbD is most powerful when implemented during process development and most effective during late-stage process development, also known as process characterisation. This involves a thorough analysis of the key operation step of the manufacturing process, where quality target product profile (QTPP) attributes are defined for a potential drug.
QTPPs outline acceptable quality ranges within which a product is both safe and efficacious. Process characterisation with QbD will also determine critical process parameters (CPPs) and their process range to determine critical quality attributes (CQAs) for an in-depth understanding of the manufacturing process. Furthermore, QbD relies on a design of experiment (DOE) approach to analyse all important factors simultaneously, which allows manufacturers to identify correlations between various components of the production process.
The ultimate goal of QbD is to manage high quality of product during lifecycle management of the drug. In this regard, CDMOs can support various drug developers through QbD.1 CDMOs work with a variety of clients, affording them a wide breadth of experience that enables them to help clients find the right solution from the outset.
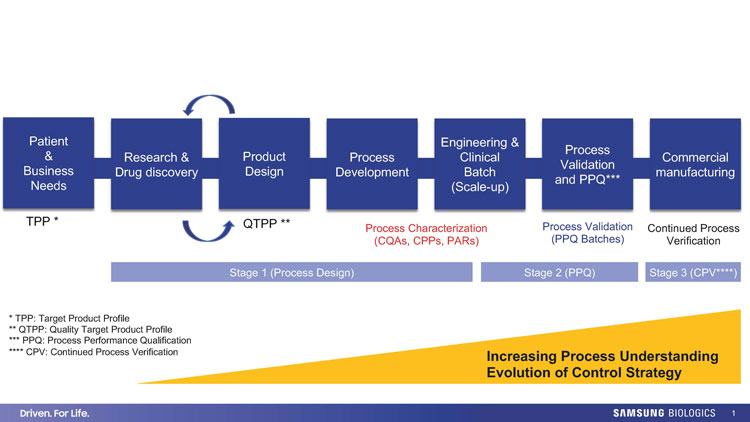

Figure 1: Biopharmaceutical product lifecycle and process validation stages; Evolution of control strategy with increased process understanding. Credit: Samsung Biologics
The cell culture process demonstrates the importance of QbD in maintaining consistent product quality over time. Some cell culture relies on complex media ingredients that can significantly affect the final product’s quality. Systematic detection and mitigation plans should be implemented to ensure a consistently high-quality drug. This includes media screening and spent-media analysis, which enable tight control of the raw material following a thorough risk assessment.
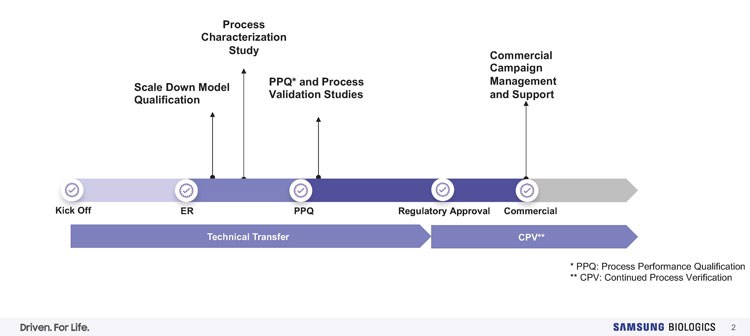

Figure 2: Tech transfer, process characterisation (PC) and process validation (PV). Credit: Samsung Biologics
The process of defining appropriate operating design space across the entire production process varies, for example, between manufacturing antibody-drug conjugates (ADCs) versus monoclonal antibodies. Navigating these challenges requires experience, process knowledge and operational flexibility. Biotech and pharma companies should look to partner with CDMOs who can implement QbD practices in a wide range of modalities and have the know-how to adapt their QbD approach accordingly.
Tech transfer for successful scaling up with QbD principles
During process development, manufacturing processes are characterised and optimised at a smaller scale. The streamlining of biopharmaceutical production workflows is done in models contained within a process development laboratory, which helps minimise waste as key bioprocesses are refined through small-scaled experimentation. However, while the reduced resource drain is helpful, the true value of these smaller scale processes lies in the veracity of their predictions. Laboratory-scale models must be representative of the larger manufacturing processes that will be employed during commercialisation – otherwise, insights gained at this stage are worth little.
QbD ensures the scale-down model has the predictive power to complete the process characterisation by regulatory agencies. Scale-down models must be qualified using statistical tools such as equivalency testing, multivariate data analysis and risk assessment, all of which lend themselves well to a QbD approach. Of course, there will invariably be differences between the scale-down model and commercial manufacturing. QbD creates a framework for identifying these differences and accounting for them as production increases.
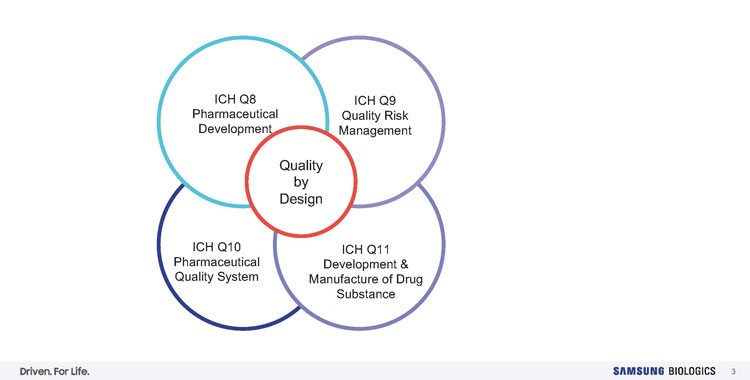

Figure 3: QbD principles and regulatory guidelines for biopharmaceutical quality management.4 Credit: Samsung Biologics
Typically, process validation is performed by using information such as criticality of process parameters from process characterisation. This validation often requires at least three consecutive batches to demonstrate that the procedure can consistently produce pharmaceutical products that meet specifications. However, process optimisation will extend through the product’s lifecycle while maintaining consistent product quality. As the commercial batch number increases, new opportunities for improvement will be discovered. By keeping QbD practices in place, including after commercialisation, companies will continually discover areas for enhancement in their manufacturing process.
In fact, the QbD approach promotes such a robust process control strategy that many regulatory bodies, such as the FDA2 and EMA, encourage implementation of QbD for risk and quality management.3 The detailed characterisation by QbD approaches ensures that safe and high-quality biologics are consistently produced. Whether companies are looking for increased confidence when filing for process validation or hunting for new efficiencies during commercialisation, QbD is a valuable strategy at every stage of a product’s lifecycle, ultimately helping to deliver well controlled and increased production of drugs to patients.
Importance of employee expertise when integrating QbD in manufacturing
Implementation of QbD principles is not the role of a single person, but rather a company-wide effort. A QbD approach requires deep knowledge of the manufacturing equipment, environment, facility, and more. Individuals with an intimate knowledge of the production process will be best equipped to provide high batch success rates throughout a product’s lifecycle, from process development to commercial manufacturing.
In fact, good technical skills can be the deciding factor in meeting deadlines. When CDMOs invest in fostering a dedicated and knowledgeable team that extends all the way from customer service to the manufacturing floor, they can find efficiencies that others might miss.
Furthermore, a strong manufacturing science and technology team means that the necessary tech transfers that come with commercialisation1 are minimally disruptive. This allows biotech and pharma company partners to achieve rapid turnaround times, while the rigorous QbD principles ensure that speed does not come at the expense of quality.
Meet the authors:




References
- Innovative Solutions For Bioprocessing Challenges: How MSAT Drives Efficient Tech Transfer. [Internet] Samsung Biologics. Available from: https://samsungbiologics.com/media/science-technology/innovative_solutions_for_bioprocessing_challenges_how_msat_drives_efficient_tech_transfer
- Guidance for Industry – Q8(R2) Pharmaceutical Development. ICH. [Internet] FDA. 2009. Available from: https://www.fda.gov/media/71535/download
- Strategic Biomanufacturing Framework And Technologies For Successful Partnership. [Internet] Bioprocess Online. 2024. Available from: https://www.bioprocessonline.com/doc/a-five-factor-framework-for-successful-cmo-partnership-0001
- Quality Guidelines – Q8, Q9, Q10 and Q11. [Internet]. Ich.org. 2020. Available from: https://www.ich.org/page/quality-guidelines
Issue
Related topics
Biopharmaceuticals, Manufacturing, Processing, Quality by Design (QbD)
Related organisations
European Medicines Agency (EMA), Samsung Biologics, US Food and Drug Administration (FDA)









