A rapid microbiological method case study for advanced therapy medicinal products
Posted: 3 July 2025 | Stacey Ramsey (Charles River Laboratories) | No comments yet
In this article, Stacey Ramsey, Senior Manager – Microbial Applications Lab, Charles River Laboratories, discusses the modernisation of rapid microbiological methods (RMMs) and the potential of ATP-bioluminescence when testing cell-based products.


Embedded as the cornerstone of microbiological quality are processes that have remained the same for over a century, unchanged from their early beginnings. To achieve optimal detectability and considerably lower risk to patients, the traditional sterility test requires a minimum of 14 days of incubation, and sometimes even longer if the test sample renders media turbid and subculturing is required to overcome visual interference.
Until recently, there was insufficient market pressure to demand a change to the drawn-out sterility test time. Traditional sterile medicines are often aseptically manufactured, and paper-based batch records can take weeks to months for a full review, depending on the batch complexity. Since the sterility test was rarely a rate-limiter in batch release, there was little appetite to warrant change.
Rapid microbial methods (RMMs) are no longer a luxury but a necessity”
Now, it appears that pharmaceutical manufacturing has turned a corner. Not only has electronic data capture become more prevalent throughout the entire manufacturing and quality testing processes, from electronic batch records, laboratory notebooks, and automated analytical testing, but the medicines themselves are evolving.
The pharmaceutical market is also tackling more challenging therapies than it targeted before, including orphan diseases, rare cancers, and infections previously thought to be untreatable. Herein lies a new challenge: for these therapies to succeed, quality control can no longer take 14 days. Rapid microbial methods (RMMs) are no longer a luxury but a necessity.
What is a rapid microbiological method?
RMMs can be broadly categorised as either growth-based or non-growth-based. While both are often utilised, growth-based methods are more common because analysing microorganisms without replication can still present challenges regarding accurate detection. These growth-based rapid methods rely on living and actively metabolising microorganisms to produce by-products that rapid systems can analyse. A popular bioanalytical method detects for the presence of adenosine triphosphate (ATP) using bioluminescence, based on the luciferin-luciferase reaction which is commonly known to provide the characteristic glow of fireflies.
ATP-bioluminescence for t-cells
Relying on microbial metabolites to find growth faster than traditional methods is challenging because it involves differentiating these metabolites from biological drug products, which are often the solutions for dealing with these complex diseases. ATP-bioluminescence is no exception: all cells produce ATP as an energy transport molecule, whether they are microbial in nature or contained within the cell therapy itself. ATP can even be a residual molecule from the biological sources used for medicine production.
Fortunately, there are ways to set microbial ATP apart from ATP produced from these biological therapies. The following case study utilised a combination of chemical depletion and filtration. During this study, nine individual lots of donor t-cells were provided for ATP-bioluminescence baseline analysis. The purpose was to determine if sterile collections of t-cell material, populated at 5.0 x 106 cells/mL, would be identified by the method as sterile.
Each sample was individually added to broth, incubated, and then analysed for residual ATP by measuring the Relative Light Unit (RLU) produced when the luciferin-luciferase reagent is added to the sample aliquot. The study found that all samples report some elevation from the baseline of negative controls, which was approximately 50-100 RLU. The highest background lot of material was lot 5, reporting around 1,000 RLU from bioluminescence, while the lowest lot reported around only 61 RLU.
This result demonstrates that while there is some residual background from the samples, and while this can vary from donor lot to lot. The sample consistently demonstrated backgrounds that were reduced compared to control studies that do not use background-ATP mitigation steps.
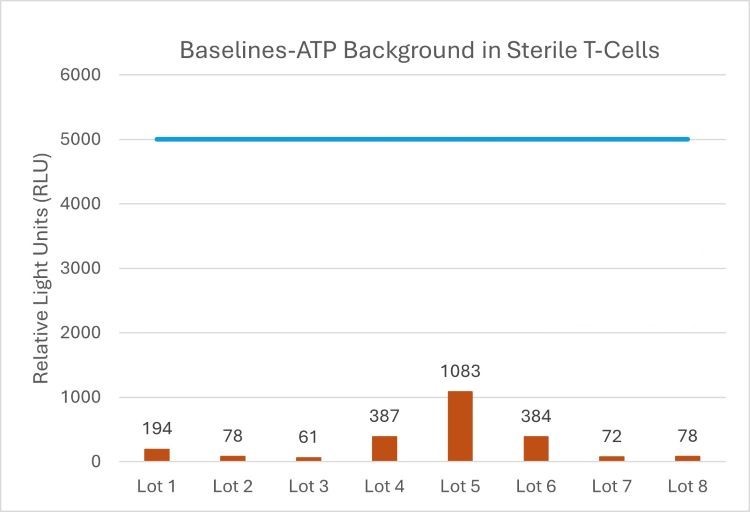

Table 1: background study using donor t-cells.
Microbial detection using ATP-bioluminescence
Following this case study, the next challenge was verifying that background ATP from cell lines can be reduced using both chemical depletion and filtration, microbial ATP detection can still be achieved. This portion of the study used standard Jurkat t-cells. Microbial inocula were individually added to the sample preparations, targeting as close to 10 CFU as possible. Each microorganism was studied in five replicates each, to verify repeatability across the experiment. Compendial sterility conditions were used, shortening the incubation period to 3-, 4-, or 5-days using ATP-bioluminescence. The study used 5,000 RLU as the cutoff for when a sample was deemed negative using this method, because this was found to be well above the highest background result reported from the baseline study.
Table 2: microbial inoculations of Jurkat t-cells.
Microorganism | CFU Level | 3-Day Celsis Adapt™ | 4-Day Celsis Adapt™ | 5-Day Celsis Adapt™ | 7-Day USP <71> | 14-Day USP <71> |
Staphylococcus aureus | 4 | 5 | 5 | 5 | 5 | 5 |
Pseudomonas aeruginosa | 6 | 5 | 5 | 5 | 5 | 5 |
Streptococcus pyogenes | 19 | 5 | 5 | 5 | 5 | 5 |
Cutibacterium acnes | 3 | 0 | 3 | 5 | 5 | 5 |
Clostridium sporogenes | 5 | 5 | 5 | 5 | 5 | 5 |
Bacteroides fragilis | 10 | 5 | 5 | 5 | 5 | 5 |
Micrococcus luteus (30-35°C) | 3 | 5 | 5 | 5 | 5 | 5 |
Micrococcus luteus (20-25°C) | 3 | 0 | 1 | 2 | 5 | 5 |
Bacillus subtilis | 10 | 5 | 5 | 5 | 5 | 5 |
Candida albicans | 8 | 1 | 1 | 5 | 5 | 5 |
Aspergillus brasiliensis | 5 | 3 | 5 | 5 | 5 | 5 |
Penicillium chrysogenum | 5 | 0 | 5 | 5 | 5 | 5 |
During this study, results show that slow-growing Cutibacterium acnes required at least four days to become positive, when inoculated with 3 CFU. C. acnes is important for this study because it is recognised as a slow-growing challenge for rapid microbial methods. It is a known cause of sterility test failures and a common human skin microorganism that cannot be typically recovered during routine environmental monitoring, increasing the importance of its recovery during routine sterility tests. When the samples were analysed using ATP-bioluminescence, the sample preparations were not visually turbid and could not be visually differentiated from the cellular sample.
Additionally, Micrococcus luteus was found to demonstrate delayed growth at 20-25°C conditions. This microorganism favours aerobic media but also prefers warmer incubation conditions. Therefore, a faster time-to-result was achieved for M. luteus by incubating within the 30-35°C condition, using Tryptic Soy Broth (TSB) as a third incubation condition.
Conclusions & future use-cases
not only are the [case study] results more rapid, but a bioanalytical result is produced instead of a subjective turbidity result”
The above summarised study is a case study in successfully utilising a rapid microbial method that may have been previously deemed incompatible with cell-based products. Through ingenuity and development, the background ATP from the human-cell line was adequately reduced, and microbial ATP was discernable from a sterile sample within five days. Further, while t-cells are commonly used in emerging cell therapy products. It is easy to see how the data could be broadly applied to various similar product types, including other mammalian or human cell lines. Finally, not only are the results more rapid, but a bioanalytical result is produced instead of a subjective turbidity result.
The implications of USP <73>
In February 2025, the United States Pharmacopoeia (USP) published its first chapter on the use of ATP-bioluminescence as a microbiological test for short shelf-life products, taking effect in August 2025. The implications of this publication are that the bar for the use of alternative methods is lowered for the products that could benefit from them the most, and in turn, benefits the patients who need therapies as quickly as possible. As part of the implementation, users no longer require alternative method validation, as these test methods have become known to be as reliable as their traditional counterparts.
This chapter’s publication indicates that regulators now find alternative microbiological methods to be increasingly repeatable and reliable, opening up the possibility of their wide applicability in the future.
Future projections
In a future state, the need will arise for rapid methods to be available for all types of [microbiological] products”
Rapid microbiology has come a long way in recent years. While ATP-bioluminescence has been researched and understood for decades, particularly in safety monitoring for foods and home and personal care industries, the rest of pharmaceutical manufacturing is now catching up. Modern manufacturing and data collection capabilities are shortening the time to release, and the need for rapid microbial results is ever-increasing. As manufacturing evolves, complacency must not remain the approach towards modernising the laboratory capabilities. It is through ongoing use, study, and regulatory review that new and modern methods will continue to shape the Quality Control environment.
In a future state, the need will arise for rapid methods to be available for all types of products, whether they are considered short shelf-life or not. Modernisation will drive objectivity and faster reaction times to quality events, improving the lives and meeting the ongoing needs of patients. This will be achieved when compendial status for rapid methods is granted for all pharmaceutical products, regardless of the product type, thereby providing products faster and with more accurate and objective test methods.
About the author


She is a QC microbiologist with 20 years of direct laboratory experience in pharmaceutical quality control. Stacey began her career in QC and R&D microbiology with CMO, Hospira (now Pfizer), in McPherson, KS, US, working with new and modern parenteral medicines. In 2015, Stacey relocated to the Carolinas, continuing her work in contract laboratory testing. Having worked in both contract manufacturing and laboratory services, she has a broad background in laboratory testing, including environmental monitoring, aseptic processing, microbial identification, endotoxin testing, and rapid microbial detection. Stacey earned her BS in Biological Sciences from Wichita State University and Master of Science degree from Friends University, US.