Calquence fails to prevent respiratory failure in COVID-19 patients
Posted: 13 November 2020 | Hannah Balfour (European Pharmaceutical Review) | No comments yet
AstraZenaca reveals Calquence (acalabrutinib) did not increase the proportion of hospitalised COVID-19 patients who remained alive and free of respiratory failure.
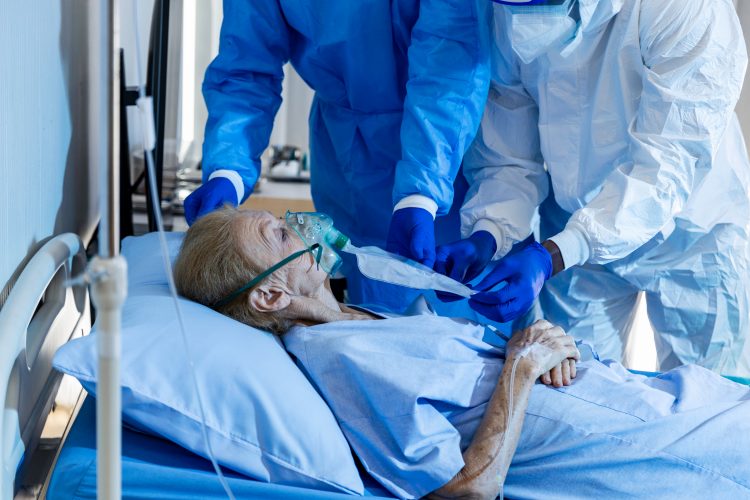

The Phase II trials evaluating Calquence (acalabrutinib) in patients hospitalised with respiratory symptoms of COVID-19 did not meet their primary efficacy endpoint. According to AstraZeneca, the addition of Calquence to best supportive care (BSC) did not increase the proportion of patients hospitalised but not on mechanical ventilation who remained alive and free of respiratory failure.
José Baselga, Executive Vice President of Oncology R&D, AstraZeneca, said: “Since the beginning of the year, AstraZeneca has been committed to doing everything we can to respond to COVID-19, including investigating existing medicines as potential treatments. The CALAVI trials were launched based on preclinical and early clinical evidence that Calquence could decrease the hyperinflammatory immune response and improve clinical outcomes in patients hospitalised with respiratory symptoms of COVID-19. While the CALAVI results are disappointing, we remain committed to advancing science that helps patients during this unprecedented global pandemic, including clinical trials for the AstraZeneca Oxford coronavirus vaccine and our long-acting antibody combination.”
The CALAVI programme comprised two Phase II trials investigating acalabrutinib plus BSC versus BSC alone in patients hospitalised with COVID-19 but not on mechanical ventilation or in the intensive care unit. The primary endpoint measured respiratory failure or death.
The safety and tolerability profiles for Calquence in the CALAVI Phase II trials were consistent with previous trials with no new safety signals reported.
Calquence
Calquence (acalabrutinib) is a next-generation, selective inhibitor of Bruton’s tyrosine kinase (BTK) being trialled in various haematologic oncology indications. In B cells, BTK signalling results in activation of pathways necessary for B-cell proliferation, trafficking, chemotaxis and adhesion. It was believed that due to the immune component in COVID-19 associated respiratory failure, Calquence may be beneficial to patients.
According to AstraZenca, the results of the CAVALI trials do not impact approved indications or pending approvals for Calquence in patients with blood cancers.
Related topics
Anti-Cancer Therapeutics, Clinical Trials, Drug Safety, Immunotherapy, Viruses




![Roche logo sign lit up [Credit: testing/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Roche-3-400x187.jpg)




