New collaboration could transform neonatal therapy
Posted: 9 January 2024 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
With no currently approved medicines for the prevention of bronchopulmonary dysplasia (BPD) in extremely pre-term infants, a new pharmaceutical collaboration could produce the first major therapeutic breakthrough for this patient group in decades.
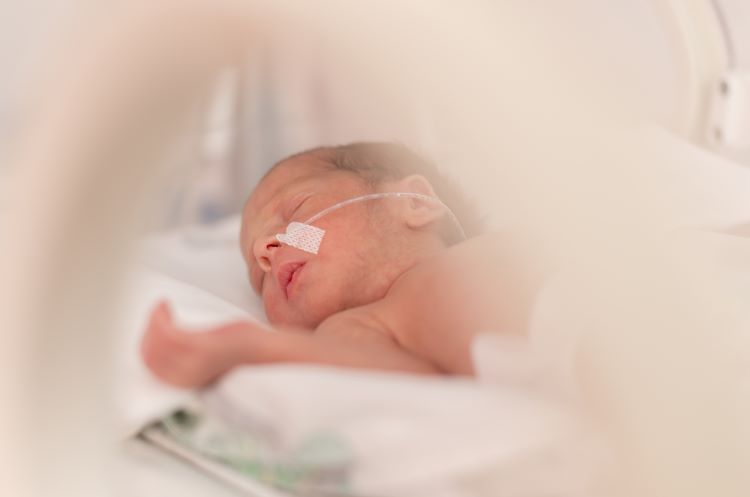

Chiesi Farmaceutici (Chiesi Group) and Oak Hill Bio, a clinical-stage neonatology and rare disease therapeutics company, have agreed to collaborate and develop, manufacture, and commercialise an investigational drug candidate for treating pre-term birth complications. OHB-607 is a human IGF-1 replacement of insulin-like growth factor-1 (IGF-1), a key driver of foetal growth and development, and its binding protein, IGFBP-3 for prevention of bronchopulmonary dysplasia in preterm infants, Chiesi explained.
Importance of the investigational treatment for pre-term infants
Until a foetus is about 30 weeks, the mother is the main source of IGF-1, which is important to note as the level of this protein is low in premature infants, due to loss of maternal source of IGF-1, Chiesi stated.
Bronchopulmonary dysplasia
Bronchopulmonary dysplasia (BPD), a chronic form of lung disease, has one of the highest unmet medical needs in the field of neonatology, according to Giuseppe Accogli, CEO of Chiesi Group. To support the immature lungs of these infants, the company highlighted that high amounts of inhaled oxygen and pressure can overstretch the alveoli. As a result, inflammation can damage the lining of the airways, the alveoli and surrounding blood vessels.
So far, clinical studies have shown that OHB-607 has the potential to significantly reduce the risk of severe bronchopulmonary dysplasia, for instance in a Phase II trial. Chiesi shared that alongside Oak Hill Bio, a Phase IIb clinical trial is planned to restart in 2024 in the US, EU and Japan.
A potential major therapeutic breakthrough
OHB-607 has the potential to be the first major therapeutic breakthrough for extremely preterm infants since lung surfactants were first approved more than 30 years ago”
“We believe that OHB-607 has the potential to be the first major therapeutic breakthrough for extremely preterm infants since lung surfactants were first approved more than 30 years ago. We hope it will lead to improved outcomes for extremely preterm infants at risk of severe bronchopulmonary dysplasia and subsequently chronic lung disease,” stated Josh Distler, President, and Chief Financial Officer of Oak Hill Bio.
Related topics
Biopharmaceuticals, business news, Clinical Development, Clinical Trials, Drug Development, Drug Manufacturing, Drug Markets, Drug Safety, Drug Supply Chain, Industry Insight, Manufacturing, Research & Development (R&D), Therapeutics









