MHRA approves bionic vision system clinical trial
Posted: 31 May 2016 | | No comments yet
Pixium Vision has received approval from the Medicines & Healthcare products Regulatory Agency (MHRA) in the UK to initiate a clinical trial for patients who have lost sight due to retinitis pigmentosa (RP) with the IRIS II bionic vision system.
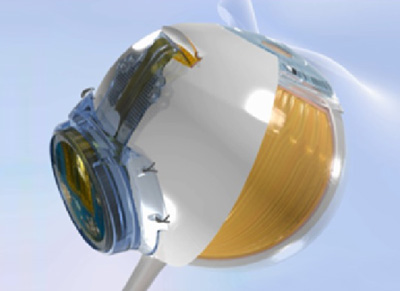

The IRIS II epi-retinal system incorporates innovative features, including a bio-inspired camera that is intended to mimic the functioning of the human eye: the imaging sensor does not take sequence of video frames with redundant information, but continuously captures the changes in a visual scene with its time independent pixels. The system also features an epi-retinal implant with 150 electrodes, almost three times more electrodes than available previously. It has an explantable design where the electrode array is secured on the retinal surface by a patented support system that allows to explant, minimising risk of retinal damage and permitting potential for upgrade to newer therapy options.
The clinical trial will be carried out at the renowned Moorfields Eye Hospital. Moorfields joins other clinical centres across Europe in participating in the trial- enabling broader patient outreach, increased opportunity to participate in the clinical trial, and paving the way for future commercialisation of the bionic vision system.
In parallel, Pixium Vision initiated last December CE mark approval process on the basis of IRIS clinical experience. Subject to CE mark approval timing, commercialisation is expected to start in H2 2016.
Pixium Vision is the only company developing an epi-retinal system for RP patients
Commenting on the upcoming trial, Mahi Muqit, PhD FRCOphth, Consultant Ophthalmologist and Vitreoretinal Surgeon at Moorfields Eye Hospital, study Principal Investigator (UK) said, “We are excited to participate in the clinical trial of IRIS II and be the first site in the UK. Patients with RP can now benefit from a new choice of retinal implant that may potentially further improve visual outcomes. This new clinical trial is key for ophthalmic reference centres like Moorfields to evaluate the latest technologies, and provide patients with a retinal implant that is differentiated and allows retinal implant exchanges in the future. We are delighted to work with Pixium Vision to develop solutions for retinal dystrophies like RP and age-related macular degeneration (AMD).”
Khalid Ishaque, CEO of Pixium Vision added, “The UK approval for the clinical study further reinforces our confidence in the IRIS II platform, our first innovative bionic vision system. Currently as the only company developing an epi-retinal system for RP patients and a sub-retinal wireless photovoltaic implant for AMD patients, we are delighted to initiate this clinical partnership with the world renowned Moorfields Eye Hospital in the UK.”