bioMérieux launches ENDOZYME® II GO, an innovative test for the detection of endotoxins in pharmaceutical microbiology control
Posted: 27 June 2018 | bioMérieux | 1 comment
bioMérieux, a world leader in the field of industrial microbiological control, announces the launch of ENDOZYME® II GO, a new endotoxin test in the bioMérieux ENDONEXT™ range of recombinant horseshoe crab Factor C (rFC) assays.
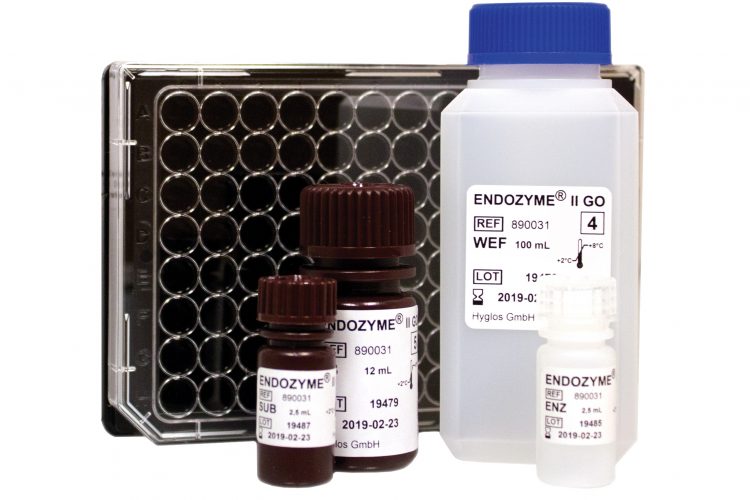

This new assay resulting from the combined expertise of bioMérieux in microbiology and Hyglos GmbH in endotoxin detection enables endotoxin testing in pharmaceutical grade water, injectable drugs and other pharmaceutical products. This new test allows an easy and fast workflow, and is particularly adapted to raw materials and final product testing.
The animal-free rFC technology, included in the European Pharmacopoeia since 2016, completely eliminates the need to harvest ecologically vulnerable horseshoe crabs endangered in Asia and protected in the United States. Their blood is used in most currently marketed tests for endotoxin detection.
“With its ultra-efficient workflow, the ENDOZYME® II GO assay, offers an alternative to further accelerate the transition to rFC of the endotoxin testing market,” said Michaël Reynier, VP Healthcare Business – Industrial Microbiology Unit at bioMérieux. “This launch is an important achievement from our development teams following the acquisition of Hyglos. Combining our respective expertise, we are strongly committed to increasing the pace of innovation in pharmaceutical endotoxin control.”
Conventional endotoxin testing requires time-consuming preparation of standard dilutions and internal controls. Such manual handling steps may further result in variability and invalid results. The new ENDOZYME® II GO assay uses the GOPLATE™ system, an innovative 96-well microplate pre-filled with required standard curve and positive product control concentrations. It is ready-to-use and enables more than 50% reduction in handling-time in comparison to conventional microplate-based endotoxin tests, as well as high precision in both standard curves and internal controls¹
bioMérieux presented first evaluation results on ENDOZYME® II GO at the 2018 PDA (Parenteral Drug Association) Annual Meeting, and will progressively roll out for worldwide distribution starting June 2018.
¹Schneider P. and al., 2018
Related topics
Assays, Drug Development, Drug Manufacturing, Microbiology, Production, QA/QC, Research & Development (R&D), Technology







![Close up view of the Merck logo on the top corner of a glass building [Credit: Michael Vi / Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Merck-aquisition-400x187.jpg)


Thanks for the terrific article