Researchers develop new method for precision drug delivery
Posted: 28 February 2019 | European Pharmaceutical Review | No comments yet
Researchers are integrating ultrasound imaging with ultrasound therapy to pave the way for a new kind of drug delivery…

The team from the James McKelvey School of Engineering and the School of Medicine at Washington University in St. Louis are developing the tools necessary for a drug delivery system, which they call cavitation dose painting.
Using focused ultrasound with its contrast agent, microbubbles, to deliver drugs across the blood-brain barrier, the research team, led by Hong Chen, assistant professor of biomedical engineering at McKelvey School of Engineering, and assistant professor of radiation oncology at the School of Medicine, was able to overcome some of the uncertainty of drug delivery.
This method takes advantage of the microbubbles expanding and contracting when they interact with the ultrasound, essentially pumping the intravenously-delivered drug to wherever the ultrasound is pointing.
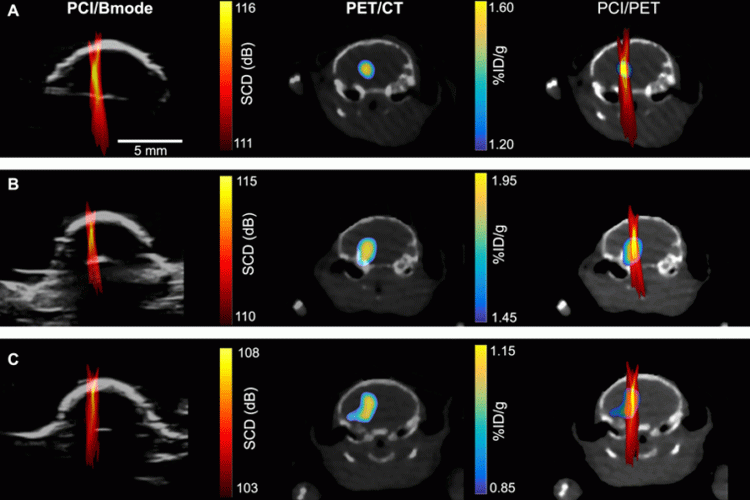
To determine where and how much of the drugs were being delivered, the researchers used nanoparticles tagged with radio labels to represent drug particles, then used positron emission tomography (PET) imaging to track their whereabouts and concentrations. They could then create a detailed image, showing where the nanoparticles were going and in what concentrations.
However, because PET imaging is expensive and associated with radioactive exposure, the team turned to passive cavitation imaging (PCI), an ultrasound imaging technique that has been under development by several groups for imaging the spatial distribution of microbubble cavitation, or the oscillation of microbubbles in the ultrasound field.
To determine whether PCI could also accurately determine the amount of drugs at a certain location, they correlated a PCI image with a PET image (which they knew can quantify the concentration of radioactive agents).
“We found there’s pixel by pixel correlation between the ultrasound imaging and the PET imaging,” said Yaoheng Yang, the lead author of this study and a second-year PhD student in the Department of Biomedical Engineering. The PCI image, therefore, can be used to predict where a drug goes and how much drug is there. Hence, she called the new technique cavitation dose painting.
Going forward, Chen believes this method could drastically change the way some drugs are delivered. Using cavitation dose painting in tandem with focused ultrasound will allow doctors to deliver precise amounts of drugs to specific locations, for example, targeting different areas of a tumour with exactitude.
“I think this cavitation dose painting technique in combination with focused ultrasound-enabled brain drug delivery opens new horizons in spatially targeted and modulated brain drug delivery,” Chen said.
Their research was published online this week in Scientific Reports.