European PFAS restriction could jeopardise pharmaceutical manufacturing
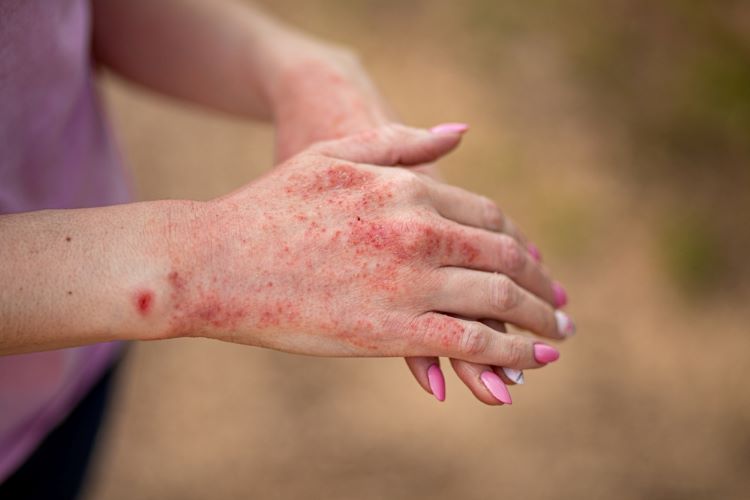
Pharmaceutical manufacturing in Europe is at risk of critical medicine shortages, states the European Federation of Pharmaceutical Industries and Associations (EFPIA), if the widest chemical substance restriction, concerning Per- and Polyfluoroalkyl Substances (PFAS), is put in place.