A reduction to practise for siRNA screening utilising high conent analysis (HCA) technologies
Posted: 10 July 2012 |
One of the major limitations of performing large-scale High Content Analysis (HCA) screens is reagent cost, indeed this fact has been a key driver in the development of assay size reduction strategies here at The Irish National Centre for High Content Screening and Analysis at Trinity College’s Department of Medicine.
As well as the obvious financial advantages of reducing assay volumes, we have also identified other key benefits to this approach, namely: Higher throughput; Improved signal to noise; Suited for the use of valuable cells, e.g. primary cells; Reduced storage and research space; Improved mixing of reagents.
The practicalities of performing cell based assays at the nano-litre scale: Despite the clear benefits to adopting miniaturisation, there are several significant barriers that must be overcome before these methods can be utilised. These are sample delivery / handling and environmental stability.
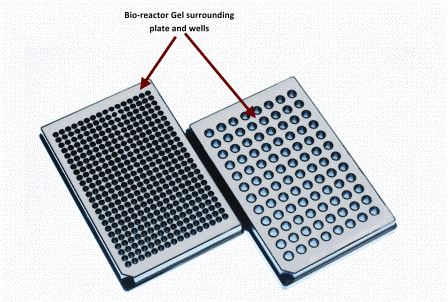

Figure 2: Showing 96 and 384 well micro-plates which have has gel based bioreactor system incorporated within the body of the plate in regions in between and surrounding the wells
One of the major limitations of performing large-scale High Content Analysis (HCA) screens is reagent cost, indeed this fact has been a key driver in the development of assay size reduction strategies here at The Irish National Centre for High Content Screening and Analysis at Trinity College’s Department of Medicine.
As well as the obvious financial advantages of reducing assay volumes, we have also identified other key benefits to this approach, namely:
- Higher throughput
- Improved signal to noise
- Suited for the use of valuable cells, e.g. primary cells
- Reduced storage and research space
- Improved mixing of reagents
The practicalities of performing cell based assays at the nano-litre scale
Despite the clear benefits to adopting miniaturisation, there are several significant barriers that must be overcome before these methods can be utilised. These are sample delivery / handling and environmental stability.
Sample delivery requirements for nano-litre scale assays
As with all cell based assays, it is necessary to perform liquid and sample preparation tasks, such as cell and reagent dispensing. As with assay preparation tasks at the nano or micro scale, it is essential that the liquid dispenser used is capable of delivering the required volumes accurately. Additionally for performing cell based assays at the nano scale, it is important that the dispensers not only permit the delivery of ultra-low volumes but also permit the accurate placement of liquid droplets in the X and Y dimensions. This functionality allows for the dispensing of multiple small volume droplets of cells and reagents into pre-defined spatial locations. It should be noted that many laboratories engaged in this type of work have successfully used fixed volume contact pin tools to perform these types of reagent dispensing tasks. However, we have found that using noncontact dispensers offers a higher degree of flexibility than the more conventionally used pin tools, although it should be noted that pin tool technologies typically permit the delivery of significantly lower volumes and a higher level of positional accuracy than many of the nano dispensing technologies currently available.
Environmental stability
Despite the availability of a large range of low volume dispensing technologies and ultra-high density micro-plates (1536 and 3072) attempts to perform assays reproducibly even at the microlitre volume ranges has in many cases has been shown to be difficult to achieve. This is in large part due to the environmental instability that is a direct consequence of the reduced capacity of a liquid at low volume to buffer against environmental fluctuations such as changes in temperature, relative humidity resulting in changes in osmolality due to moisture loss through evaporation and local changes in the partial pressures of atmospheric gases such as CO2 resulting in changes in pH. This poor environmental stability in turn can lead to edge effects in micro-plates resulting in reduced experimental reproducibility.
Our approach
To address these issues we have developed a novel methodological approach and a new technology that is robust and permits us to routinely perform cell based assays at volumes ranging from 50 – 100 nanolitres.
To achieve this we routinely perform our sample delivery tasks using acoustic dispensing technology (Labcyte Echo©). This low volume dispensing device has allowed us to perform a wide range of liquid handling steps that previously would have been achieved by using multiple devices. Additionally this technology has sufficiently good X/Y resolution to permitting multiple liquid spotting tasks at the same location, hence allowing the delivery of siRNA and transfection reagent followed by addition of cells all easily within a 200 micrometre target.
Environmental stability
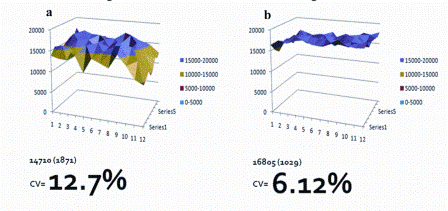

Figure 1: Showing the consequences of edge effects on cell growth in STD 96 well plates (a) Vs 96 well plates modified with an environmental buffering bio-reactor system (b) It will be noted that environmentally buffered plates results in a lower coefficient of variation (cv cell number). A549 cells were incubated under standard tissue culture conditions (37°C 5% CO2 >95% Relative humidity) for 7 days, cells were then stained with the nuclear dye Hoechst and then counted using High Content Analysis
To compensate for the issues surrounding the environmental stability, we have developed a new bioreactor technology that retains heat and CO2 and prevents evaporation, hence reducing environmental fluctuations and reducing edge effects (Figure 1).


Figure 2: Showing 96 and 384 well micro-plates which have has gel based bioreactor system incorporated within the body of the plate in regions in between and surrounding the wells
This bioreactor technology can be incorp – orated either into traditional micro-titre plate formats (Figure 2) or into a micro well array device (Figure 3). This bioreactor technology has been developed into a solid gel, which surrounds the wells of the plates, but does not in any way interact with the contents of the well. The gel retains heat and CO2 and prevents evaporation by maintaining high levels of humidity locally within and above the wells of the micro-plate.
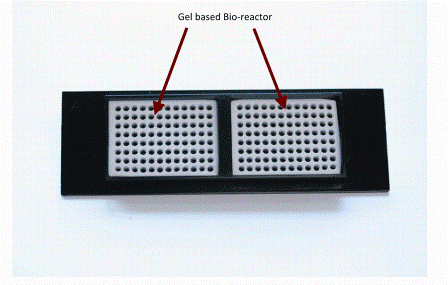

Figure 3: Showing a specialised miniaturised 2 X 96 well array device with environmental buffering technology surrounding the wells (white). This array technology has been mounted into a standard microscope slide footprint
The assay work flow for nano scale siRNA screening using High Content Analysis
The assay work flow can be subdivided into three distinct processes:
(i) Arraying of siRNA molecules – This task is achieved by spotting a complex siRNA and transfection mixture, for example into wells of the micro-plate or onto the surface of the micro-array slide. For this task, we have utilised acoustic dispensing technologies. Typically, we have dispensed volumes ranging from 50 – 100 nL of this mixture, once delivered to the target, spotted reagents are left at room temperature until dry (see Figure 4)
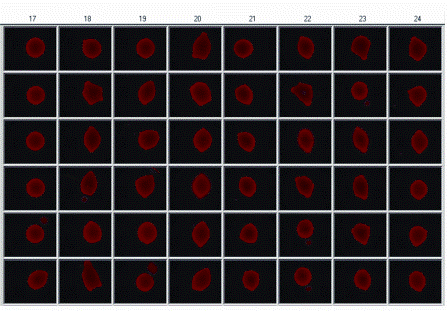

Figure 4: Showing a portion of a micro-plate array of printed with 50 nL droplets comprising of fluorescently labelled siRNA and lipid transfection reagent complex. Droplets were delivered by acoustic dispensing and then dried at room temperature and stored until required
(ii) Cell dispensing and cell incubation – To achieve an effective transfection, cells must be placed directly on top of the dried spotted siRNA transfection reagent complex (Figure 5). Typically, 50 – 300 cells are dispensed in 100 nL of buffer and incubated with the transfection complex for a minimum period of 24 hours. Once this incubation step has been completed, wells were back-filled with culture media and further incubated for a further 48 – 72 hours to allow sufficient time for gene silencing to occur
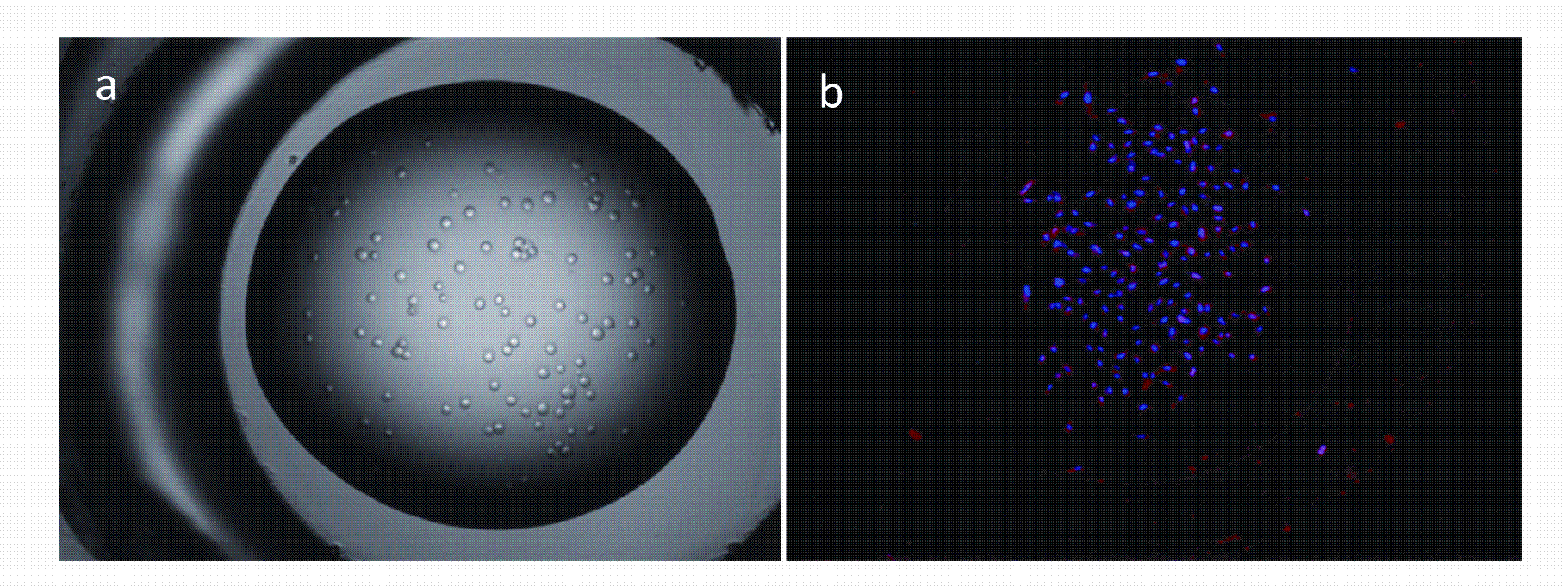

Figure 5: (a) Showing a 100uL droplet of cells in media freshly dispensed into a well of a micro array device using acoustic dispensing technologies. (b) Showing uptake of fluorescently labelled siRNA (red) into cells (nuclei stained in blue) after a 72 hour incubation with labelled siRNA/ lipid transfection complex
(iii) Image acquisition and analysis – Once cell treatment and incubation procedures have been completed, the next step is to analyse cells (Figure 6). For miniaturised cellular assay, formats as described above, we have chosen High Content Analysis as an assay output. High Content Analysis holds advantages over the more traditional methods such as colorimetric or luminescence based assay endpoints which are often destructive, require relatively large sample size, are non-contextual and provide only limited information, whereas image based techniques such as HCA are nondestructive, only require small samples, provide information in the context of the whole cell and information rich multiparametric outputs.
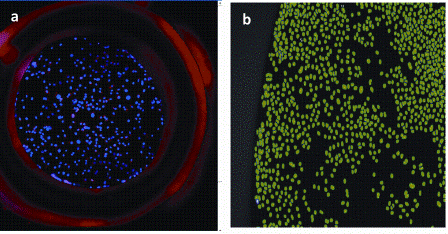

Figure 6: Image acquisition and Analysis of cell based assays performed in micro well array format (a) Showing X4 image of cells labelled with the DNA dye Hoechst in a well of a micro-array device (b) Showing a 10X image of Hoechst stained nuclei which have been detected and masked (green) by a simple High content image analysis algorithm for determining nuclear count per sample
Data from proof of concept studies performed at nano litre volumes
To test this new approach we performed early proof concept studies we performed using a few siRNAs with well characterised effects on cell growth and cell survival (Figure 7). These consisted of a (i) non targeting siRNA (as a negative control) (ii) an siRNA molecule targeting the gene surviving (BIRC5) which we found to be mildly toxic to A549 cells in previous experiments using conventional reverse transfection methods at conventional assay volumes (iii) we also used a proprietary Tox siRNA which has been specifically designed to induce cell death in cells and is commonly used as a transfection control and finally an siRNA targeting (iv) Kiff 11 because of its potent cytotoxic effects. The cell number data shown in Figure 7 was found to be very close to that found in previous studies where cell based assays were performed using con ventional reverse transfection methods at 25 microlitre volumes.
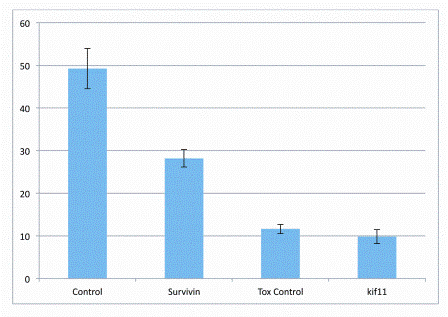

Figure 7: The effect of a collection of siRNA controls on cell number Preliminary data from a proof of principle study where cells were spotted onto pre-complexed siRNA arrayed into environmentally buffered high density micro well plates. The following siRNA’s were spotted using acoustic dispensing technologies (i) non targeting siRNA (ii) Survivin (BIRC5), Tox siRNA and (iv) Kiff 11. 100 nL of cell suspension was then spotted directly on to the siRNA targets. Cells were then incubated for 24 hours prior to back filling with an excess of media, cells were then maintained in culture for a further 48 hours prior to analysis
Conclusion
In this article, we have described a study to assess the feasibility of utilising assay miniaturisation approaches in siRNA screens. To achieve this, we have utilised state-of-the-art acoustic liquid handling technologies, High Content Analysis and a novel bioreactor system that buffers the assay environment against evaporation, and fluctuations of temperature and atmospheric gasses, hence enabling assays to be routinely performed at volumes as low as 100nL. Conversely, although this approach does offer substantial benefits for both cost saving and logistical benefits e.g. increase throughput and the potential of using rare or expensive cell types such as primary or stem cells for large scale screens. However, it should also be noted that the equipment setup costs for such an approach would be very high and out of the reach of many laboratories and institutions.
About the author
Dr Davies received his PhD in molecular Cardiology from the Faculty of Medicine, University of Dundee Scotland. Following the completion of this research project he joined Tayside Institute of Child Health, Dundee, Scotland where he focused on the development of specialized cell based assays and perfusion systems for the study of hypoxic and ischemic insult in cardiac muscle cells and tissues. Dr Davies undertook further research in the IBLS (University of Glasgow) where he focused on the development of cell based assays and living cellular arrays for use with high throughput and high content screening of cardiac cells. Dr Davies now holds a senior position as Director of high content research facility (Trinity HCA) Department of Clinical Medicine Trinity College Dublin.
Issue
Related topics
Environmental Monitoring, HCA (High Content Analysis), siRNA