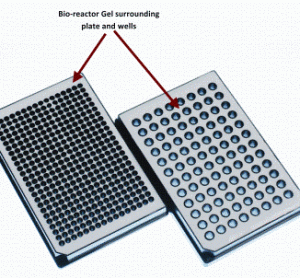
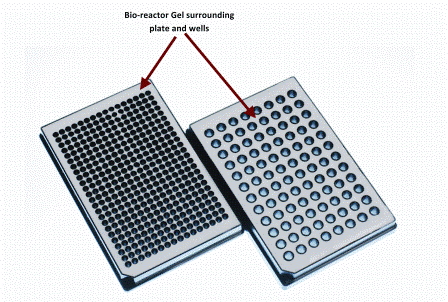
A reduction to practise for siRNA screening utilising high conent analysis (HCA) technologies
10 July 2012 | By Anthony Mitchell Davies & Anne Marie Byrne, Department of Clinical Medicine Trinity College Dublin; Holger Erfle, BIOQUANT-Zentrum Ruprecht-Karls-Universität Heidelberg; Graham Donnelly, Rita Murray & Peadar MacGabhann, Biocroi Ltd
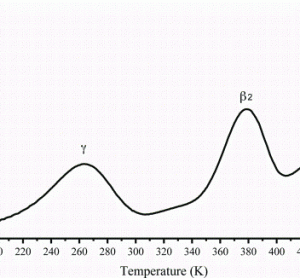
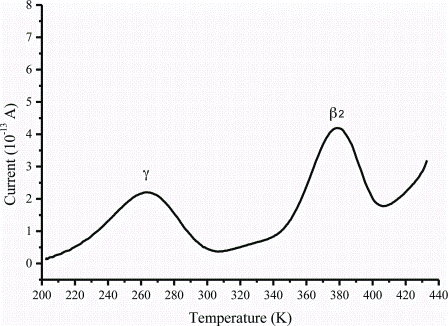
One of the major limitations of performing large-scale High Content Analysis (HCA) screens is reagent cost, indeed this fact has been a key driver in the development of assay size reduction strategies here at The Irish National Centre for High Content Screening and Analysis at Trinity College’s Department of Medicine.…