Biomarkers and the tumour microenvironment
Posted: 7 February 2009 | Brett Hall, Biomarkers Team Leader, Johnson & Johnson | No comments yet
The current cost of developing a new medicine for the treatment of human disease has been estimated at $1 to $2 billion (€750-1.5 million1,2). Given progressive increases in the cost of developing new drugs, pharmaceutical companies are facing significant pressure to streamline discovery methods and increase the translational efficiency of their newly discovered compounds.
The current cost of developing a new medicine for the treatment of human disease has been estimated at $1 to $2 billion (€750-1.5 million1,2). Given progressive increases in the cost of developing new drugs, pharmaceutical companies are facing significant pressure to streamline discovery methods and increase the translational efficiency of their newly discovered compounds.
The current cost of developing a new medicine for the treatment of human disease has been estimated at $1 to $2 billion (€750-1.5 million1,2). Given progressive increases in the cost of developing new drugs, pharmaceutical companies are facing significant pressure to streamline discovery methods and increase the translational efficiency of their newly discovered compounds.
Further complicating pharmaceutical processes are:
- An expectation for a new drug to display significant improvements in clinical outcomes over standard of care
- Dramatic reductions in drug-related side effects (i.e., targeted therapies)
- Erosion of public trust in large pharmaceutical companies
- Cumbersome regulatory oversight (e.g., FDA and EMEA)
- Exposure to emerging markets where initial costs will swell due to new regulatory challenges, establishment of intellectual property rights, reductions in drug reimbursement levels, and variations in disease incidence.
Pharmaceutical leaders are faced with the question of how to overcome these hurdles and thrive in a global economy in recession.
Part of the solution can rely on a successful biomarker strategy, which can have a profound impact on the clinical success of a compound by identifying pharmacodynamic (PD) biomarkers that validate drug response in the clinic. Additionally, biomarkers can target a patient population, which is more likely to respond to a given drug (predictive biomarkers), and thereby reduce pharmaceutical costs associated with drug development. For example, recognition that a drug is not impacting its intended clinical target (i.e., PD biomarkers) or that a given group of patients have compensatory genetic lesions that will increase or decrease the effectiveness of a new drug (i.e., predictive biomarkers) can save a company hundreds of millions of dollars that it costs to run Phase III clinical trials. In this review, I will focus on how a better understanding of the tumour microenvironment can impact oncology-related biomarker strategies and reduce financial costs associated with development of new medicines.
The journey from discovery to Phase III clinical trials
Evaluation of human tumour cell lines in 2D tissue culture, multi-well plates continues to be the workhorse for high-throughput drug screens in Oncology R&D. While this process has produced the majority of the oncology drugs in the market, the failure rate on the path from early discovery to the clinic remains unacceptably high. For example, for every 100,000+ compounds tested, approximately 5,000 to 10,000 may display a favorable signature (e.g., inhibition of tumor cell line growth or induction of tumor cell death)2. After further in vitro screening, less than 250 of these compounds will likely be selected for pre-clinical evaluation, and of those, many will have an unfavorable toxicity profile or fail to demonstrate reasonable efficacy in preclinical animal models2. Lead optimisation of the “best” compounds might produce five to ten drugs that display the correct balance of in vivo efficacy, favorable pharmacokinetics (PK), and a acceptable toxicity profile. In Phase I human clinical trials, a lead compound’s PK, PD, tolerability, toxicity, and maximum tolerated dose (MTD) are scrutinised, and while clinical response is not typically required for a successful Phase I clinical trial, many lead compounds fail at this point due to unfavorable toxicity, tolerability, or lack of drug activity based on PD biomarkers. Patient recruitment is then expanded for Phase II trials and specific disease indication(s) are selected. Clinical expectations during Phase II include significant improvement in clinical response over standard of care (SoC), a proportional response within the patient population, and an acceptable safety and toxicity profile at the effective dose. Disease indications for Phase II clinical trials are selected based on preclinical biomarker data, which include in vitro tissue culture, in vivo preclinical animal model systems, and, if present, clinical responses observed in Phase I. At completion of Phase II clinical trials, the decision to proceed to Phase III (i.e., the “go/no-go” decision) is often the most difficult decision a pharmaceutical company will make during compound development, with respect to financial risk. A Phase III clinical trial alone can cost hundreds of millions of dollars to run2, and barring a stellar clinical responses across a large percentage of the patient population during Phase II trials (i.e., a blockbuster drug), the decision to move into Phase III remains a formidable task.
Biomarkers: discovery to preclinical development
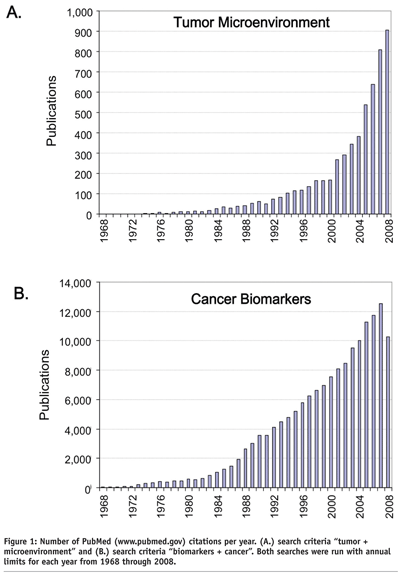
Over the past two decades, a growing number of studies within oncology have focused on the tumor microenvironment (TME) and biomarkers (see Figure 1). The TME can be generally defined as the dynamic and complex environment, both cellular and non-cellular, that a tumour cell experiences while in its natural state within the host. The complexity, structure, dimensionality, biological impact, and pathophysiology of the TME can have profound effects on tumour cell growth rates3,4, gene expression profiles5-7, physiological response to paracrine and autocrine growth factors8 (e.g., reference4 (3D/IL-6) vs. reference9 (2D/IL-6)), cell:cell and cell:extracellular matrix (ECM) interactions10, motility/metastasis/ invasion11,12, tumour-like signaling networks5, sensitivity/resistance to cytotoxic drugs13, and redefining tumourigenicity itself4,14. With a better understanding of how the TME impacts tumour cell behavior, improvements in preclinical assays have been and continue to be paramount to better model tumour cell behavior in the context of an in vivo TME15.

Pharmaceutical drug discovery currently utilises in vitro model systems that have inherent strengths such as familiarity, low cost, extensive literature base, ease of use, and high-throughput screening capability. However, many in vitro models harbour critical shortcomings with respect to in vivo tumour biology. These issues can drastically mislead drug discovery efforts and contribute to high drug development failure rates. For example, the standard practice growing and evaluating tumour cells in two dimensions (2D), on hard plastic dishes, in the presence of potent prenatal growth factors (i.e., fetal bovine serum (FBS)) fundamentally lacks the dimensionality, complexity, and stiffness of the TME found in vivo. Devoid of a functional in vivo-like TME predictably results in numerous biological anomalies, which include: artificially high tumour cell doubling times3,16, significant alterations in tumour cell gene expression profiles7, loss of correlation in predicting tumor-drug sensitivity and resistance13, and a lost opportunity to identify compounds that specifically target the tumor cell and/or the TME, in a normal biological context15 (see Figure 2).
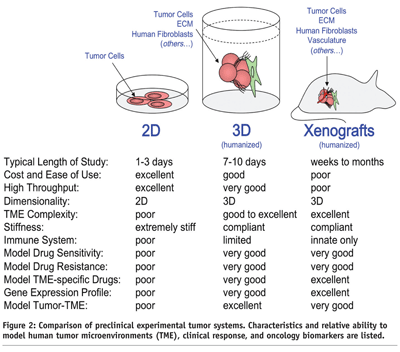

The formation of human tumour xenografts, following injection of human tumour cells into immunocompromised rodents, remains a central preclinical in vivo model for drug development and compound lead optimisation. Similar to 2D in vitro models, xenografts have inherent strengths and weaknesses. Strengths include an in vivo TME that provides a good approximation to a human TME with respect to dimensionality, stiffness, and ECM complexity. However, there are several weaknesses of xenografts when modeling drug responsiveness. Some of the notable TME-related deficiencies include, a dysfunctional immune system, abnormally high tumour growth rates, and a species-TME barrier (see Figure 2). More specifically, xenografts tend to grow much quicker than human tumours, which make cytotoxic agents appear to be more effective in preclinical animal models. Also, human xenograft tumours suffer from an incomplete TME as a consequence of the species barrier that exists between mouse and man (i.e., many tumour-TME factors are able to cross talk, while other factors have limited or a complete dysfunction16. Because of the species barrier, scientists tend to migrate toward use of human tumour cell lines that “grow well” in rodent models, but in doing so, we have unintentionally selected for the most aggressive and autocrine of our human tumor cell lines (e.g., MDA-MB-231 (breast), PC3 (prostate), U87 (brain), RH30 (muscle), SKOV-3 (ovarian), HT29 (colon), A549 (lung)). While at first glance, this seems to be a reasonable trade-off, there remains an obvious divide between the human condition, where it is more likely that a cancer patient harbours a tumour that fully interacts with and depends on its TME. Experimentally, these phenomena can be illustrated by comparing two of the most commonly cited human-derived breast cancer cell lines, MDA-MB-231 and MCF-7. The MDA-MB-231 cell line (ERα negative adenocarcinoma17) is considered “highly aggressive and metastatic”. In contrast, the MCF-7 cell line (ERα positive adenocarcinoma18) is considered “less aggressive and non-metastatic”. These views stem from an extensive collection of data that was compiled using primarily 2D and xenograft preclinical model systems. The biological paradox of this vantage point (i.e., between preclinical models and the human condition) stems from the fact that both of these cell lines were isolated from women who harboured fully metastatic disease, both lines were established from pleural effusions (i.e., both lines were isolated from “aggressive tumours” in their human hosts), and both women ultimately perished from metastatic disease. Interestingly, if the TME is “humanised” by the addition human fibroblasts isolated from the breast or bone, MDA-MB-231 or MCF-7 display similar growth kinetics3,4,19 and invasiveness12, and neither estrogen supplementation nor Ras mutation is required for MCF-7 xenograft engraftment and growth4,12. However, these tumour characteristics are only observed in preclinical models that closely mimic the dynamic and complex nature of 3D TME.
Biomarkers – preclinic to clinic and back again
The transition of a sound biomarker strategy into Phase I and subsequent clinical trials relies on a vast body of data compiled during drug discovery, early development, and preclinical lead optimisation. Thus, it is essential that Discovery Team and Biomarker Team goals are strongly aligned and that experimental models utilised during preclinical efforts replicate human cancer, as closely as possible. It is also essential that the Biomarker Team communicate with Clinical Teams to coordinate and obtain a robust set of patient tissue samples for PD and predictive biomarker assessment. Even the best biomarker strategy will fail if adequate tissue samples are not collected, processed, stored, shipped, and evaluated in an efficient and timely manner. While PD biomarkers are of the highest priority during Phase I and II clinical trials, predictive “responder/non-responder” biomarker strategies should be well developed by the end of Phase II clinical trials. Biomarker data from clinical samples, which validate or discredit clinical drug efficacy, ensures that the best possible outcome of a clinical trial is known.
Conclusions
Given the myriad of TME-related issues surrounding the most commonly used preclinical models in oncology drug discovery and development, it is clear that current experimental approaches that are associated with high failure rates during transitions from 2D tissue culture to xenografts and from xenografts into the clinic, need to be improved upon. Consistent with the current “TME-deficient” preclinical model systems, we have a strong propensity to discover lead compounds that are cytotoxic and/or inhibit cellular proliferation in both tumour cell (therapeutic) and normal (toxicity) populations. If we are to improve compound discovery success rates in the face of mounting fiscal pressures, we must integrate and model more complex, in vivo-like, TME into our preclinical and ex vivo model systems. And, central to a seamless transition from preclinical data to the clinic (and back), there must be a robust and multifaceted biomarker strategy that is based on both tumor cells and their microenvironment. If the models and assays used for biomarker assessment, both PD and predictive, during clinical trials do not reflect the human condition (e.g., dimensionality, complexity, stiffness, and cross-talk of a human-like TME), they will unlikely yield high quality biomarker data for both preclinical models and clinical samples. In conclusion, we have an exciting new journey ahead of us in oncology drug development, and it is becoming increasingly evident that our efforts must consider the impact of the human TME on tumor cell behavior.
References
- Adams,C.P. & Brantner,V.V. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff. (Millwood.) 25, 420-428 (2006).
- Masia N Bureau of International Information Programs. U. S. DEPARTMENT OF STATE http://us.info.gov/, (2006).
- Sasser,A.K., Mundy,B.L., Smith,K.M., Studebaker,A.W., Axel,A.E., Haidet,A.M., Fernandez,S.A., & Hall,B.M. Human bone marrow stromal cells enhance breast cancer cell growth rates in a cell line-dependent manner when evaluated in 3D tumor environments. Cancer Lett. 254, 255-264 (2007).
- Sasser,A.K., Sullivan,N.J., Studebaker,A.W., Hendey,L.F., Axel,A.E., & Hall,B.M. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J 21, 3763-3770 (2007).
- Kass,L., Erler,J.T., Dembo,M., & Weaver,V.M. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem. Cell Biol. 39, 1987-1994 (2007).
- Ghosh,S., Spagnoli,G.C., Martin,I., Ploegert,S., Demougin,P., Heberer,M., & Reschner,A. Three-dimensional culture of melanoma cells profoundly affects gene expression profile: a high density oligonucleotide array study. J Cell Physiol 204, 522-531 (2005).
- Kenny,P.A., Lee,G.Y., Myers,C.A., Neve,R.M., Semeiks,J.R., Spellman,P.T., Lorenz,K., Lee,E.H., Barcellos-Hoff,M.H., Petersen,O.W., Gray,J.W., & Bissell,M.J. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 1, 84-96 (2007).
- Miralem,T., Steinberg,R., Price,D., & Avraham,H. VEGF(165) requires extracellular matrix components to induce mitogenic effects and migratory response in breast cancer cells. Oncogene 20, 5511-5524 (2001).
- Badache,A. & Hynes,N.E. Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res. 61, 383-391 (2001).
- Cukierman,E., Pankov,R., & Yamada,K.M. Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol. 14, 633-639 (2002).
- Cukierman,E., Pankov,R., Stevens,D.R., & Yamada,K.M. Taking cell-matrix adhesions to the third dimension. Science 294, 1708-1712 (2001).
- Studebaker,A.W., Storci,G., Werbeck,J.L., Sansone,P., Sasser,A.K., Tavolari,S., Huang,T., Chan,M.W., Marini,F.C., Rosol,T.J., Bonafe,M., & Hall,B.M. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 68, 9087-9095 (2008).
- Fiebig,H.H., Maier,A., & Burger,A.M. Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur. J Cancer 40, 802-820 (2004).
- Ancrile,B., Lim,K.H., & Counter,C.M. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 21, 1714-1719 (2007).
- Weigelt,B. & Bissell,M.J. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin. Cancer Biol. 18, 311-321 (2008).
- Rangarajan,A. & Weinberg,R.A. Opinion: Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat. Rev Cancer 3, 952-959 (2003).
- Cailleau,R., Young,R., Olive,M., & Reeves,W.J., Jr. Breast tumor cell lines from pleural effusions. J Natl. Cancer Inst. 53, 661-674 (1974).
- Soule,H.D., Vazguez,J., Long,A., Albert,S., & Brennan,M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl. Cancer Inst. 51, 1409-1416 (1973).
- Karnoub,A.E., Dash,A.B., Vo,A.P., Sullivan,A., Brooks,M.W., Bell,G.W., Richardson,A.L., Polyak,K., Tubo,R., & Weinberg,R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557-563 (2007).




