QbD and PAT: From Science to Compliance
Posted: 30 July 2009 | | No comments yet
Boards of health like the Food and Drug Administration and European Medicines Agency and ICH guidelines Q8, Q9 and Q10, provide a framework for Quality by Design (QbD) that fully integrates drug substance and drug product development with the principles of Quality Risk Management (QRM), Process Analytical Technology (PAT) and Quality Systems (QS). QbD may begin as early as drug substance engineering and could extend to formulation and process development.
QbD combined with Process Analytical Technology (PAT) tools enable process control and increase the assurance that product quality attributes are achieved consistently, and/or that manufacturing efficiencies are obtained.
In recent years Quality by Design (QbD) and Risk and Science Based Initiatives have been the topics of many meetings and conferences within the pharmaceutical community. The new paradigm of building quality into the process and product, as opposed to policing quality in the final dosage form, is fast becoming the norm, particularly for new products in the pipeline. Scientific based quality analytical technologies are finding their way from the lab to the manufacturing line. But even though the science is proven, the path to implementation is not so clear.
Synthetic chemistry research and development teams have been using QbD and PAT principles for decades. These principles have been named differently throughout the years and have been championed by many different business or quality drivers. Industries such as food, semi-conductors, oil and many more, owe their profit and very existence to the QbD and PAT principles.
A strategy for PAT implementation in a QbD environment has to be developed in order to achieve the final goal of process control. The challenge is not demonstrating proof of concept or reliability; the real challenge is integrating PAT into a QbD system that assures quality and continuous process improvement in a commercial manufacturing process. This system should assure not only the product quality, but also should guarantee integrity of the data and compliance.
QbD and PAT
QbD: “A systematic approach to development that begins with predefined objectives and emphasises product and process understanding and process control, based on sound science and quality risk management.” ICH Q8(R1, 2008)
PAT is one of the many tools or enablers of QbD. PAT can be an invaluable tool through life cycle management. During product and process development it can enhance prior knowledge and improve process understanding, help with process mapping and monitoring, model building and along with QRM, help establish a design space and a control strategy. During manufacturing operations PAT can help ensure process robustness and consistent output, as well as enabling operational flexibility through adaptive process controls, based on process understanding, and ultimately Real Time Release (RTR) through a science/risk based approach and Quality Systems. For continual improvement, PAT tools, such as multivariate data analysis and process control systems, enable historical data tracking and trending for continual improvement and consistent patient outcome.
From PAC to PAT
PAC: Center for Process Analytical Chemistry CPAC, established at the University of Washington in 1984:
A consortium of Industrial, National Laboratory and Government Agency Sponsors addressing multidisciplinary challenges in Process Analytical Technology (PAT) and Process Control through fundamental and directed academic research.
PAC: “The goal of process analytical chemistry is to supply quantitative and qualitative information about a chemical process. Such information can be used not only to monitor and control a process, but also to optimise its efficient use of energy, time, and raw materials.” Callis, Illman, Kowalski (1987).
PAT: “The Agency considers PAT to be a system for designing, analysing, and controlling manufacturing through timely measurements (i.e., during processing) of critical quality and performance attributes of raw and in-process materials and processes, with the goal of ensuring final product quality.” Food and Drug Administration PAT Guidance (2004).
Terminology
The language of QbD and PAT is in constant flux. Control space to control strategy, Design Space or Normal Operating Ranges (NOR), Proven Acceptable Ranges or (PAR), Experience Space, Knowledge Space, Continuous Improvement to Continual Improvement, Multivariate Data Analysis (MVDA) or Chemometrics…and the list goes on. Practitioners should have a clear understanding of the terminology.
When it comes to MVDA and Chemometrics, the list of acronyms increases exponentially and is influenced by the software package involved and the scientist experience.
PAT misconceptions
A true PAT platform should provide timely measurements! PAT is Dynamic, Real Time and Process Based, with the capability of process correction and potential open or close loop control. The latter would be the ideal scenario. Close loop process controls are old school to process/control engineers in other industries, but pharma industry has a long way to go.
Spectroscopy Alone is Not PAT! Even though spectroscopic techniques such as Raman and NIRS can be part of a Holistic QbD approach, they are considered chemical and physical characterisation techniques when they are not in a dynamic real time production environment.
Early adopter’s disappointments of new techniques, concomitant failures of implementation and lack of understanding of the true nature of QbD and PAT, have added challenges to QbD and PAT development and implementation.
Champion, goal, value and opportunities
Champion: QbD and PAT need Champions. QbD and PAT can not be developed, implemented and sustained as a part time effort. It has to be a serious commitment supported by management in order to achieve an effective cultural transformation.
Goal: It has to be identified, commonly accepted/acknowledged by the team, and embraced by the organisation as desirable, achievable and realistic. Some of the previous PAT’s unfulfilled promises are the worst obstacles to overcome.
The goal of PAT development and implementation should be aligned with the scope of a QbD plan. A PAT system is developed to measure critical process parameters and critical quality attributes, understand product and process variability, and thus control manufacturing processes to help achieve a predefined target product profile and/or bring robustness to the process.
The flow diagram in Figure 1 depicts the variability existing from raw materials, processes, intermediates to final product. A successfully implemented PAT system should be able to:
- Identify, understand and manage the sources of variability;
- Establish relationship between raw material, process parameters and final product quality attributes;
- Control raw material/processes to ensure CQAs as specified.
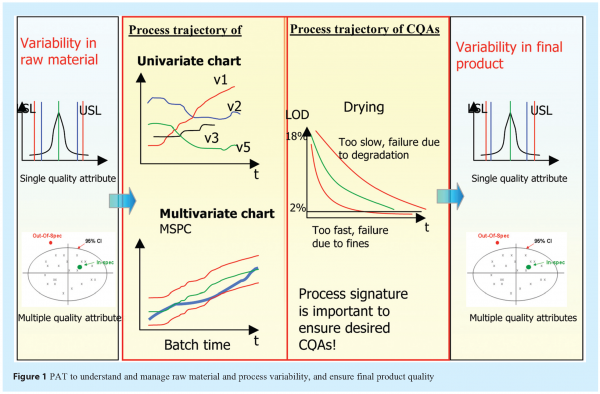

Variability of a single quality attribute of in-coming raw material can be represented using a normal distribution with specification limits, while a combination of multiple quality attributes of raw material can be summarised in a multivariate chart, e.g. PCA Scores plot. Pharmaceutical processes are multivariate by nature, with many variables impacting processes and product quality attributes. It is important to monitor and evaluate batch process performance by modeling multiple variables simultaneously, instead of looking at each variable individually. Multivariate control chart, e.g. Scores control chart, derived by multivariate statistical process control (MSPC), can be used to represent a process signature or fingerprint of the process, and detect/diagnose faults as the batch evolves. What is often neglected is that process trajectory of a CQA of intermediate is also important. For instance, drying too fast or too slow during the drying process may lead to batch failure. With all upstream variability well understood and managed, final product quality attributes can then be controlled as specified.
The authors recommend that PAT teams are engaged early on in the process development stages, in order to understand and evaluate the critical process parameters and quality attributes. For existing products, PAT efforts could be directed to processes that have shown a need for improvement. Do not do PAT for the sake of PAT.
Value and opportunities: ROI considerations initial cost might be high, but the cost of quality is priceless. At the end of the day, the cost of a sensor and allied IOQ and implementation will be justified by the first batch saved or recall averted. Build a business case, do the maths and take it upstairs. If there is not a champion, ask for one! Again, do not do PAT for the sake of PAT.
Utilisation of PAT in developing process understanding and control
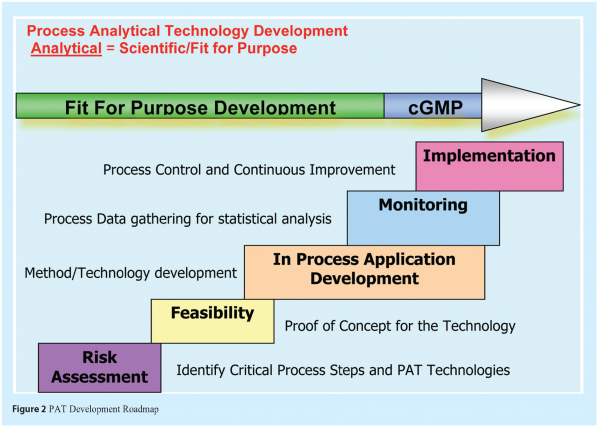
Early PAT development schemes had four stages,
- Feasibility
- Development
- Monitoring
- Implementation.
A QbD aligned PAT development scheme should involve a Quality Risk Assessment (QRA) as the first step. And it should meet some basic scientific and potential regulatory requirements.
The PAT development roadmap should consist of key elements such as;
- risk assessment
- selection and evaluation of the feasibility of the appropriate PAT tool
- deployment and in-process application development
- monitoring, data collection and analysis
- implementation.
The Risk Assessment should identify critical unit processes, critical process parameters (CPP), critical quality attributes (CQA) and potential mitigation and control strategies that may include the use of PAT. A number of tools for risk assessment are available, from qualitative to quantitative. ICH Q9, Quality Risk Management provides guidance on this subject.
Feasibility should identify the likelihood of one or more solutions/technologies meeting the desired Business/Technical requirements. The feasibility may also involve further assessments to define the potential risks, opportunities, alternatives, and solution/recommendations for the PAT tool development and implementation. The assessment should be based on an outline design of system requirements in terms of Input, Processes, Output (IPO) and Procedures. The outcome of the feasibility will be confirmed by the solution of the intended use of the method implementation. It is important to mention that the feasibility study is preferably performed in a developmental or R&D environment.
Application Development may require Design of Experiments (DoE). A DoE is a structured, organised method for determining the relationship between factors that affect the process and the output of that process. The DoE may be performed in two scales; (1) laboratory (small) and (2) commercial scale (large).
During the in-process application development, the method should be optimised and scientifically validated. That is, tested for the merits of a validation like: specificity, linearity, range, accuracy, precision, repeatability, intermediate precision, robustness, detection limit, quantitation limit, etc. to ensure that the method is fit for purpose. The term scientifically validated, in this context, refers to a validation in an R&D non-GMP environment in order to assess method feasibility and appropriateness.
Method deployment at scale may still require an Application Development approach using DoE. Scale up and equipment changes may be factors to consider, as well as commercial scale supply sourcing related variability.
Monitoring: The method validation in a cGMP compliant environment usually takes place during the monitoring stage.
Plant site quality systems may impact PAT deployment and monitoring even under “Safe Harbor” when monitoring data will not have an impact on commercial, clinical or development batch disposition.
For Implementation, an Implementation Team and a Validation Team should be assembled to categorise the implementation and validation requirements and propose acceptance criteria for each stage, based on the application or intended use of the PAT Tool and method. The intended use of each could be established during the DoE developed for the method. These requirements and criteria will be ultimately in a validation protocol and described in the validation report. The acceptance criteria must be in-line with the expected specification, protocol requirements, development experience and manufacturing practice.
Cultural Change, Education and Infrastructure
QbD, QRM, PAT etc have to be understood, accepted and embraced as the new ways of working. No more Quality by Inspection! A QbD culture will require champions, trainers and educators and empowered operators, supervisors, engineers, IT/S and quality personnel. It is everyone’s responsibility to get on board.
New SOPs, guides and skill sets will be required. The mindset has to change from a culture of fixed unalterable processes, to adaptive process controls, science and risk based processes and regulatory flexibility. Many technical business units have to be engaged in the process. Engineering, information technology, physical characterisation and statistics teams (to mention a few) should be incorporated in the PAT groups. Again, it is everyone’s responsibility to get on board.
PAT at Wyeth
At Wyeth, QbD and PAT has been a cross functional and inter-departmental effort across business and product units. The approach has been to replicate successful implementation strategies and technology platforms in order to reduce learning curves, improve manufacturing efficiencies and harmonise technology platforms and quality systems.
Wyeth has a consistent, well-defined, systems approach to QbD that is being implemented for all new products across the manufacturing divisions, aligned with R&D as part of a holistic QbD approach.
Regulatory Aspects
Early communication with the Boards of Health (BOH) and engaging them as partners in this new paradigm is key to successful implementation. In the end, no surprises, better understanding and co-development are all critical components to positive outcomes.
Utilisation of PAT in process control and continual improvement
This is the final frontier! Real Time Release (RTR), continual improvement and other regulatory or financial benefits may be initially driven by the desire of increasing quality, decreasing cost and improving manufacturing.
There are several ways to look at this new paradigm. Should we do all or most of the PAT and process understanding during development and implement manufacturing/ engineering process controls during manufacturing? Or should we identify and implement PAT in manufacturing to control and optimise? The possibilities are endless, it is in many ways unchartered territory and the pharmaceutical industry and the BOH are working on a path forward.
The main challenge at this stage is the development and deployment of the Quality Systems to support QbD and PAT in full compliance in a GMP environment.