Antibody-based proteomics to study cellular signalling networks
Posted: 19 March 2008 | Jan van Oostrum, Head of Business Development, Zeptosens, a division of Bayer (Schweiz) AG and Hans Voshol, Group Leader Protein Sciences, Novartis Institutes for BioMedical Research | No comments yet
The complexity of drug discovery faces many challenges; principally, the failure of drug candidates during the development process as a result of adverse effects or lack of efficacy. A key reason for this high attrition rate is that we are only just beginning to understand the complexity of the response(s) from a biological system to perturbations, such as a disease state or drug treatment. Subsequently, a deeper insight into the molecular mechanisms underlying both disease processes and drug action will ultimately contribute to increased productivity through the drug discovery process[1,2].
The complexity of drug discovery faces many challenges; principally, the failure of drug candidates during the development process as a result of adverse effects or lack of efficacy. A key reason for this high attrition rate is that we are only just beginning to understand the complexity of the response(s) from a biological system to perturbations, such as a disease state or drug treatment. Subsequently, a deeper insight into the molecular mechanisms underlying both disease processes and drug action will ultimately contribute to increased productivity through the drug discovery process[1,2].
The complexity of drug discovery faces many challenges; principally, the failure of drug candidates during the development process as a result of adverse effects or lack of efficacy. A key reason for this high attrition rate is that we are only just beginning to understand the complexity of the response(s) from a biological system to perturbations, such as a disease state or drug treatment. Subsequently, a deeper insight into the molecular mechanisms underlying both disease processes and drug action will ultimately contribute to increased productivity through the drug discovery process[1,2].
In recent years, progress has been made in ascribing pathological conditions to defects in molecular pathway components, for example, linking dysregulation of signalling pathways to cancer and inflammatory diseases.
Kinases and phosphatases are key regulators in signalling pathways, so it is not surprising that across the pharmaceutical industry, a substantial percentage of drug discovery efforts are focused on targeting these enzyme classes. In particular, the modulation of cellular kinase activities, which is one of the most rapidly growing areas in the development of novel drugs.
Understanding the information flow through signalling networks and how these can best be manipulated to halt or redirect the flow of aberrant signalling is a challenging endeavour. The initial step would be to describe the full complexity of signalling networks at a molecular level, including activities specific to a particular cell type, such as dynamic feedback mechanisms, pathway crosstalk, signalling kinetics and of course pathway activation states in normal and disease situations.
For a kinase pathway, the information flow, or pathway flux, predominantly depends on the ratio of phosphorylated and non-phosphorylated protein species reflecting the activation state of the biological system. If cellular activity is compared over time, i.e. at various stages of disease progression, or before, or after drug treatment, it is likely that correlations can be found between the activation, biological and disease states. Small molecules that modulate the activity of signalling proteins are useful tools to dissect the functional roles and connections of the individual nodes in a pathway3.
Using such a ‘systems approach’, one can begin to build a model that will not only provide a contextual understanding of the molecular mechanisms of disease, but also has the potential to facilitate the validation of therapeutic modulation of regulatory and metabolic networks4. A direct consequence of such an approach would be the early recognition of off target and side effects of drug candidates, as well as the identification of putative biomarkers.
Phosphoproteomics
Different types of tools are available for the analysis of protein phosphorylation, an area of biochemistry often referred to as phosphoproteomics. Mass spectrometry, in conjunction with the enrichment of phosphopeptides or proteins, has developed into a key method and has resulted in studies describing thousands of phosphorylation sites. Protein phosphorylation occurs mostly with three amino acids: serine (Ser), threonine (Thr) or tyrosine (Tyr). Ser and Thr sites comprise just over 99% of phosphorylations.
Nevertheless, there is also a strong interest in tyr-phosphorylation because a significant number of key receptors, such as the EGF receptor family, display tyr-kinase activity upon ligand binding. The enrichment of tyr-phosphopeptides is facilitated by the availability of sequence-independent antibodies, while ser-phosphopeptides and thr-phosphopeptides usually undergo metal affinity purification. Mapping of phosphotyrosine residues is also offered as a commercial service termed PhosphoScan® by Cell Signaling Technologies5.
While mass spectrometry is the method of choice for discovery in phosphoproteomics, as yet it is not the optimal solution for assaying known phosphorylation sites in a large number of samples.
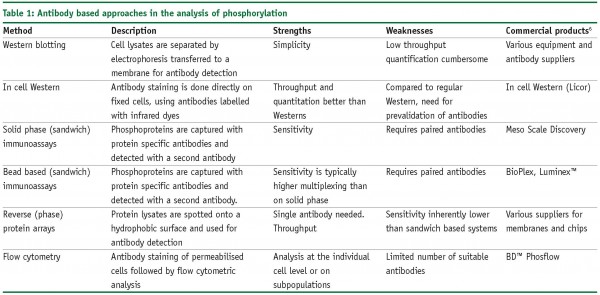
Sample preparation is fairly laborious, difficult to automate and in most cases requires relatively large sample amounts (>1 mg of protein/sample). Novel targeted mass spectrometry methods are currently being developed to address this issue, but screening applications will continue to be dominated by antibody based approaches for the foreseeable future. The phosphorylation status of signalling pathway components can be measured using sequence specific anti-phosphoprotein antibodies that specifically recognise the phosphorylated isoforms of such kinase substrates. Therefore, the activity status of multiple signalling pathways can be probed through parallel phosphospecific analysis. These antibodies can be utilised in a wide variety of formats, ranging from the ‘good old’ Western blot to advanced microarray-based platforms. Table 1 provides an overview of the most commonly used approaches.

Due to their minute sample consumption, reverse protein microarrays enable truly multiplexed analysis by replicating the same sample many times on separate arrays. This type of array, in which a protein extract is immobilised and queried with antibodies or other reagents that bind to a specific protein in the sample, is often referred to as reverse protein microarray (RPA). Opposed to this, forward arrays are those in which the capture reagent (e.g. the antibody) is immobilised7. Forward arrays have the inherent advantage of a better sensitivity and owing to the capture step, which enriches the analyte substantially. Reverse arrays require only a single and not a matched pair of antibodies, hence saving significantly on assay development time.
Reverse protein arrays
The simplest yet still widely used form of a reverse protein arrays is the dot blot, in which small volumes of protein solutions are applied to a membrane, either manually or using a small vacuum manifold. Gradually, the technology evolved with the advent of nitrocellulose coated slides8 and spotters.
Much of the work in the reverse array field has been focused on analysing phosphorylation states in human cancer tissue, to unravel the longer term signalling mechanisms underlying human cancer8. However, the scalability and capacity of the latest generation reverse protein array systems opens the way to profiling pathways almost in ‘real-time’, providing the necessary basis to model such dynamic systems.5
A key requirement for such phospho-specific screening data to feed into pathway models is the quantitative aspect. Among the different proteomics technologies that are suitable for that purpose, we describe here a reverse array platform based on using the planar waveguide technology for significantly improved sensitivity. Planar waveguide reverse protein arrays make it feasible to obtain reproducible and quantitative protein expression information about the dynamic aspects of cell signalling. Samples are titrated in serial dilutions (Figure 1) on the array to ensure that the assay is linear and therefore relative or absolute (using an internal standard) quantitation is possible. For this platform, cells or tissue samples are subjected to a one step extraction using denaturing conditions, under which the potentially labile protein phosphorylations are effectively frozen. Figures 2 and 3 show selected results obtained with our reverse protein microarrays.
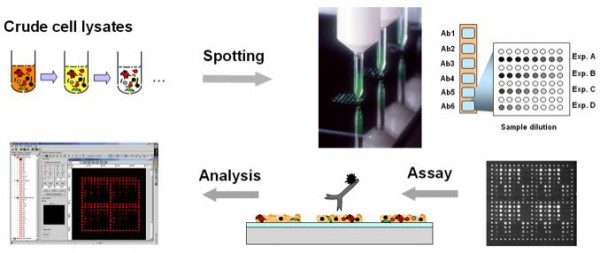

Figure 1: Crude cell lysate samples, which are produced with a denaturing solution, are spotted into arrays onto specially prepared glass chips by non-contact spotting. The samples are probed, for example by fluorescently labelled antibodies.
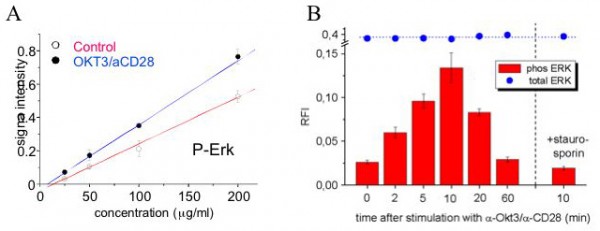

Figure 2: Monitoring phosphorylation events using reverse protein array technology: The human T-cell leukaemia line Jurkat was either stimulated with OKT3 and anti-CD28 antibodies (blue) or treated with control medium (red). p44 / 42 MAPK (Erk), a key node in T-cell signalling, is instantly activated upon stimulation, as shown by the rapid increase in phosphorylation at Thr202/Tyr204 (Figure 2, A and B). For every sample (e.g. time point) is stained with the anti-phospho antibody, and then plotted against the spotted protein concentration (Figure 2, A). By ensuring linearity of the measurement, the slope of the obtained curve can be used to obtain relative quantification (Figure 2, B). This example illustrates how reverse protein arrays can capture highly dynamic signalling events.
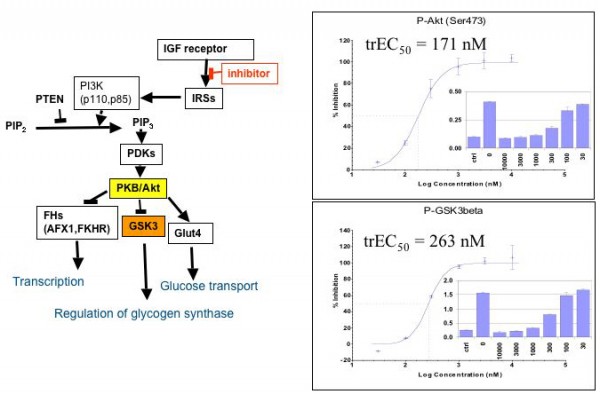

Figure 3: Monitoring the downstream effect of cell signalling inhibitors: starved A431 cells were stimulated with insulin and co-treated with increasing concentrations of an inhibitor of the IGF1receptor tyrosine kinase. After 30 minutes of treatment, the cells were lysed and the phosphorylation levels of Akt and GSK3 were monitored with antibodies specific for; Ser473-Phospho-Akt and Ser9-phospho-GSK3. By plotting the percent inhibition versus inhibitor concentration, one can derive EC50 like data from such experiments, termed trEC50 for signalling transduced downstream EC50 values.
ZeptoChips® use planar waveguide technology for improved sensitivity and are made of thin film planar waveguides, consisting of a 150 nm thin film of a material with high refractive index (e.g. Ta2O5), which is deposited on a transparent glass support with lower refractive index. A laser light beam is coupled into the wave guiding film by a diffractive grating that is etched into the glass. The light propagates within this film and creates a strong evanescent field perpendicular to the direction of propagation into the adjacent medium. The field strength decays exponentially with the distance from the waveguide surface and its penetration depth is limited to approximatly100 nm. Upon fluorescence excitation by the evanescent field, excitation and detection of fluorophores is restricted to the sensing surface, while signals from unbound molecules in the bulk solution are not detected. This results in a significant increase in the ‘signal-to-noise’ ratio, compared to conventional optical detection methods.
A typical microarray (Figure 4) has space for six arrays, each comprising of 352 spots. Each array has four columns of spots, used to calibrate the energy loss when the light travels across the waveguide. Typically, 32 samples are spotted in four dilutions, ensuring one always remains within the linear part of the binding curve and then in duplicates. If the concentration of the analytes is known, then the number of dilutions can be reduced. Each array will be probed with one antibody. Each spot will have a volume of around 0.5 nl (ø100µm) and will contain the amount of protein contained in a single cell. Spotting is performed by a non-contact piezoelectric spotter, with a spotting capacity of about 360 arrays, which enables 360 antibodies can be probed in one overnight spotting run. After printing, chips are blocked with albumin (as done for Western blots), and can be stored in this blocked state for over a year at 4°C.
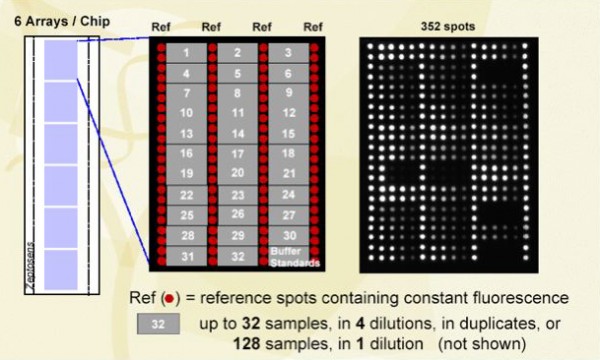

Figure 4: From chips to arrays to spots
Due to the high sensitivity and high throughput capability of the reverse protein array approach, it will be feasible to obtain protein expression profiles and signalling pathway information on a wide variety of cell lines and tissue samples. Interesting applications include the comparative analysis of signalling pathway(s) events in normal versus diseased tissue, the comparative analysis of protein expression in various systems, also the elucidation of the dynamic aspects of pathway events and the profiling of compounds to reveal signalling and cross-pathway effects of drug candidates (Figure 5). In addition, analysis of healthy versus diseased tissue (including animal models) will provide insights into the pathways underlying pathologies and provide a platform for molecular diagnostics. In a future approach, the screening of body fluids with a reverse array approach may enable the investigation a large number of individual body fluid samples for a limited set of proteins contained in them, to establish variations in protein expression levels.
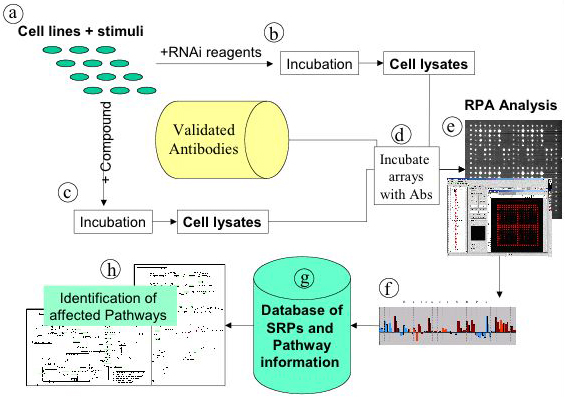

Figure 5: Possible application of reverse protein arrays in systems biology: structure pathway activity relationship (SPAR). Selected cell lines are treated with appropriate combinations of activating stimuli (a), and treated with either si/shRNA (b) or test compounds (c). Treated cells are sampled in a time dependent manner and lysed before being spotted on reverse protein arrays. The arrays are incubated with pre-defined antibodies (d), measurements are taken (e). The systems response profiles (SRPs) are deduced from the fluorescence intensities (f) and stored in a database (g) along with pathway information. The treatment with siRNAs allows identification of SRPs caused by well-targeted network perturbations, which can serve as the reference set against which SRPs caused by drug candidates can be compared. Thereby off target effects can be deduced (h).
References
- Butcher, E.C., Berg, E.L., Kunkel, E.J.: Systems biology in drug discovery. Nature Biotech 2004, 22:1253-1259.
- Fishman, M.C., Porter, J.A.: A new grammar for drug discovery. Nature 2005, 437:491-493.
- Sevecka, M., MacBeath, G.: State based discovery: a multidimensional screen for small molecule modulators of EGF signaling. Nature Methods 2006, 3:825-831.
- Cho, C., Labow, M., Reinhardt, M., van Oostrum, J. Peitsch, M.C.: The application of systems biology to drug discovery. Current Opinion in Chemical Biology 2006, 10:294-302.
- Cell Signalling Technologies Inc.
- References for applications: Phosflow: bdbiosciences.com, In-Cell Western: licor.com, Meso Scale: meso-scale.com, Luminex: luminexcorp.com, Bioplex: bio-rad.com.
- Haab, B.B: Antibody arrays in cancer research. Mol Cell Proteomics 2005, 4:377-383.
- Sheehan K.M et al., Use of reverse phase protein microarrays and reference standard development for molecular network analysis of metastatic ovarian carcinoma. Mol Cell Proteomics 2005, 4:346-355.
Jan van Oostrum
Head of Business Development, Zeptosens, a division of Bayer (Schweiz) AG
Jan van Oostrum was recently appointed as Head Business Development at Zeptosens and is the former Head of the Protein Science and Technology Unit at the Novartis Institutes for Biomedical Research in Basel, Switzerland. Jan van Oostrum received his Ph.D. from Columbia University in New York and holds an affiliate faculty appointment at the Department of Anatomy and Cell Biology at the McGill University in Montreal. His main interest is on integration and application of protein microarray technologies in order to obtain a ‘systems’ perspective on diseases linked to molecular signalling pathways.
Hans Voshol
Group Leader Protein Sciences, Novartis Institutes for BioMedical Research
Hans Voshol holds an MSc in Biochemistry and a PhD in Bio-Organic Chemistry from Utrecht University (The Netherlands). After a postdoctoral fellowship at the Institute of Neurobiology of the Federal Institute of Technology (Zürich, Switzerland), he joined Novartis Basel in 1996. His current position is that of group leader Protein Sciences at the Novartis Institutes BioMedical Research. His research interests are in the field of proteomics of signalling pathways.




